Tacrolimus-induced cholestatic hepatotoxicity after renal transplantation: a case report
- PMID: 38409063
- PMCID: PMC10898131
- DOI: 10.1186/s13256-024-04394-6
Tacrolimus-induced cholestatic hepatotoxicity after renal transplantation: a case report
Abstract
Background: In this manuscript, we report a case of tacrolimus-associated hepatotoxicity in a kidney transplant recipient.
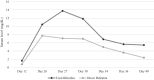
Case presentation: In this case report, a 56 years old Arab male patient who received a kidney transplant presented with icterus, weakness, and lethargy two weeks after transplantation and tacrolimus initiation. In laboratory analysis hyperbilirubinemia and a rise in hepatic enzymes were observed. All possible causes of hepatotoxicity were examined. The panel for infectious causes was negative. Drug-induced liver injury was diagnosed. The patient's immunosuppressive regimen was changed to a cyclosporine-based regimen and after this change bilirubin and hepatic enzymes decreased and the patient was discharged without signs and symptoms of hepatitis.
Conclusion: It seems that the patient's hyperbilirubinemia was due to tacrolimus, and the patient's bilirubin decreased after stopping tacrolimus.
Keywords: Hepatotoxicity; Hyperbilirubinemia; Tacrolimus.
© 2024. The Author(s).
Conflict of interest statement
The authors declared none.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical