Differential pulmonary toxicity and autoantibody formation in genetically distinct mouse strains following combined exposure to silica and diesel exhaust particles
- PMID: 38409078
- PMCID: PMC10898103
- DOI: 10.1186/s12989-024-00569-7
Differential pulmonary toxicity and autoantibody formation in genetically distinct mouse strains following combined exposure to silica and diesel exhaust particles
Abstract
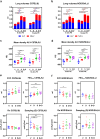
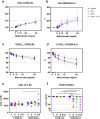
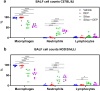
Background: Inhalation of airborne particulate matter, such as silica and diesel exhaust particles, poses serious long-term respiratory and systemic health risks. Silica exposure can lead to silicosis and systemic autoimmune diseases, while DEP exposure is linked to asthma and cancer. Combined exposure to silica and DEP, common in mining, may have more severe effects. This study investigates the separate and combined effects of occupational-level silica and ambient-level DEP on lung injury, inflammation, and autoantibody formation in two genetically distinct mouse strains, thereby aiming at understanding the interplay between genetic susceptibility, particulate exposure, and disease outcomes. Silica and diesel exhaust particles were administered to mice via oropharyngeal aspiration. Assessments of lung injury and host response included in vivo lung micro-computed tomography, lung function tests, bronchoalveolar lavage fluid analysis including inflammatory cytokines and antinuclear antibodies, and histopathology with particle colocalization.
Results: The findings highlight the distinct effects of silica and diesel exhaust particles (DEP) on lung injury, inflammation, and autoantibody formation in C57BL/6J and NOD/ShiLtJ mice. Silica exposure elicited a well-established inflammatory response marked by inflammatory infiltrates, release of cytokines, and chemokines, alongside mild fibrosis, indicated by collagen deposition in the lungs of both C57BL/6J and NOD/ShilLtJ mice. Notably, these strains exhibited divergent responses in terms of respiratory function and lung volumes, as assessed through micro-computed tomography. Additionally, silica exposure induced airway hyperreactivity and elevated antinuclear antibody levels in bronchoalveolar lavage fluid, particularly prominent in NOD/ShiLtJ mice. Moreover, antinuclear antibodies correlated with extent of lung inflammation in NOD/ShiLTJ mice. Lung tissue analysis revealed DEP loaded macrophages and co-localization of silica and DEP particles. However, aside from contributing to airway hyperreactivity specifically in NOD/ShiLtJ mice, the ambient-level DEP did not significantly amplify the effects induced by silica. There was no evidence of synergistic or additive interaction between these specific doses of silica and DEP in inducing lung damage or inflammation in either of the mouse strains.
Conclusion: Mouse strain variations exerted a substantial influence on the development of silica induced lung alterations. Furthermore, the additional impact of ambient-level DEP on these silica-induced effects was minimal.
Keywords: Autoimmunity; Diesel exhaust particles; Lung inflammation; Mice; Silica; Silicosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Update of
-
Differential Pulmonary Toxicity and Autoantibody Formation in Genetically Distinct Mouse Strains Following Combined Exposure to Silica and Diesel Exhaust Particles.Res Sq [Preprint]. 2023 Oct 9:rs.3.rs-3408546. doi: 10.21203/rs.3.rs-3408546/v1. Res Sq. 2023. Update in: Part Fibre Toxicol. 2024 Feb 27;21(1):8. doi: 10.1186/s12989-024-00569-7. PMID: 37886437 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

