Comprehensive analysis of mitochondria-related genes indicates that PPP2R2B is a novel biomarker and promotes the progression of bladder cancer via Wnt signaling pathway
- PMID: 38409085
- PMCID: PMC10898125
- DOI: 10.1186/s13062-024-00461-6
Comprehensive analysis of mitochondria-related genes indicates that PPP2R2B is a novel biomarker and promotes the progression of bladder cancer via Wnt signaling pathway
Erratum in
-
Correction: Comprehensive analysis of mitochondria-related genes indicates that PPP2R2B is a novel biomarker and promotes the progression of bladder cancer via Wnt signaling pathway.Biol Direct. 2024 Apr 18;19(1):27. doi: 10.1186/s13062-024-00474-1. Biol Direct. 2024. PMID: 38637864 Free PMC article. No abstract available.
Abstract
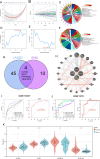
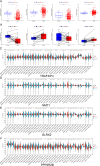
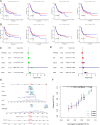
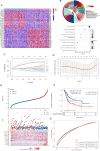
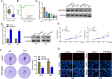
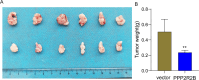
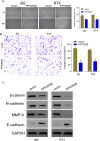
Bladder cancer (BC) is the fourth and tenth most common malignancy in men and women worldwide, respectively. The complexity of the molecular biological mechanism behind BC is a major contributor to the lack of effective treatment management of the disease. The development and genesis of BC are influenced by mitochondrial retrograde control and mitochondria-nuclear cross-talk. However, the role of mitochondrial-related genes in BC remains unclear. In this study, we analyzed TCGA datasets and identified 752 DE-MRGs in BC samples, including 313 down-regulated MRGs and 439 up-regulated MRGs. Then, the results of machine-learning screened four critical diagnostic genes, including GLRX2, NMT1, PPP2R2B and TRAF3IP3. Moreover, we analyzed their prognostic value and confirmed that only PPP2R2B was associated with clinical prognosis of BC patients and Cox regression assays validated that PPP2R2B expression was a distinct predictor of overall survival in BC patients. Them, we performed RT-PCR and found that PPP2R2B expression was distinctly decreased in BC specimens and cell lines. Functional experiments revealed that overexpression of PPP2R2B distinctly suppressed the proliferation, migration and invasion of BC cells via Wnt signaling pathway. In summary, these research findings offer potential molecular markers for the diagnosis and prognosis of BC, with the discovery of PPP2R2B particularly holding significant biological and clinical significance. This study provides valuable clues for future in-depth investigations into the molecular mechanisms of BC, as well as the development of new diagnostic markers and therapeutic targets.
Keywords: Biomarker; Bladder cancer; Metastasis; Mitochondrial-related genes; PPP2R2B; Wnt signaling pathway.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures


References
-
- Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J, Siegel RL. Cancer treatment and survivorship statistics, 2022. CA 2022;72(5):409–436. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

