Microbial composition, functionality, and stress resilience or susceptibility: unraveling sex-specific patterns
- PMID: 38409102
- PMCID: PMC10898170
- DOI: 10.1186/s13293-024-00590-7
Microbial composition, functionality, and stress resilience or susceptibility: unraveling sex-specific patterns
Abstract
Background: Following exposure to traumatic stress, women are twice as likely as men to develop mood disorders. Yet, individual responses to such stress vary, with some people developing stress-induced psychopathologies while others exhibit resilience. The factors influencing sex-related disparities in affective disorders as well as variations in resilience remain unclear; however, emerging evidence suggests differences in the gut microbiota play a role. In this study, using the single prolonged stress (SPS) model of post-traumatic stress disorder, we investigated pre- and post-existing differences in microbial composition, functionality, and metabolites that affect stress susceptibility or resilience in each sex.
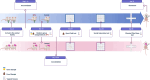
Methods: Male and female Sprague-Dawley rats were randomly assigned to control or SPS groups. Two weeks following SPS, the animals were exposed to a battery of behavioral tests and decapitated a day later. Based on their anxiety index, they were further categorized as SPS-resilient (SPS-R) or SPS-susceptible (SPS-S). On the day of dissection, cecum, and selected brain tissues were isolated. Stool samples were collected before and after SPS, whereas urine samples were taken before and 30 min into the SPS.
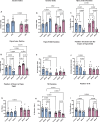
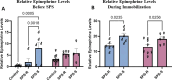
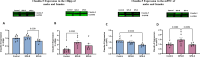
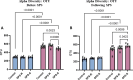
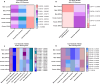
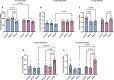
Results: Before SPS exposure, the sympathoadrenal axis exhibited alterations within male subgroups only. Expression of tight junction protein claudin-5 was lower in brain of SPS-S males, but higher in SPS-R females following SPS. Across the study, alpha diversity remained consistently lower in males compared to females. Beta diversity revealed distinct separations between male and female susceptible groups before SPS, with this separation becoming evident in the resilient groups following SPS. At the genus level, Lactobacillus, Lachnospiraceae_Incertae_Sedis, and Barnesiella exhibited sex-specific alterations, displaying opposing abundances in each sex. Additionally, sex-specific changes were observed in microbial predictive functionality and targeted functional modules both before and after SPS. Alterations in the microbial short-chain fatty acids (SCFAs), were also observed, with major and minor SCFAs being lower in SPS-susceptible males whereas branched-chain SCFAs being higher in SPS-susceptible females.
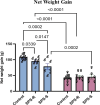
Conclusion: This study highlights distinct pre- and post-trauma differences in microbial composition, functionality, and metabolites, associated with stress resilience in male and female rats. The findings underscore the importance of developing sex-specific therapeutic strategies to effectively address stress-related disorders. Highlights SPS model induces divergent anxiety and social behavioral responses to traumatic stress in both male and female rodents. SPS-resilient females displayed less anxiety-like behavior and initiated more interactions towards a juvenile rat than SPS-resilient males. Sex-specific pre-existing and SPS-induced differences in the gut microbial composition and predictive functionality were observed in susceptible and resilient rats. SPS-resilient males displayed elevated cecal acetate levels, whereas SPS-susceptible females exhibited heightened branched-chain SCFAs.
Keywords: Anxiety; Microbiome; Resilience; SCFA; SPS; Sex differences.
Plain language summary
After experiencing traumatic stress, women are more likely than men to develop mood disorders like anxiety and depression. However, people's responses to trauma vary—some develop mental health issues while others remain resilient. Recent research suggests that the bacteria in the gut might play a role in these differences. In this study, using a rat model of post-traumatic stress disorder (PTSD), we investigated whether there are differences in gut bacteria between male and female rats before and after stress exposure. The study involved two groups of rats—one not exposed to stress (control) and the other exposed to a traumatic event (stressed). The rats' behavior was evaluated using different tests to determine who among the males and females were vulnerable to stress and who were resilient. We found that even before the stress, there were differences in the types of bacteria and their functions in the guts of male and female rats. These differences were also linked to how they responded to stress. Interestingly, the bacteria that were more abundant in resilient males were found to be more abundant in vulnerable females. Additionally, the traumatic stress affected these bacteria and the substances they produce differently in males and females. In essence, our study demonstrates that the types of gut bacteria, their functions, and their products contribute to stress resilience in different ways for male and female rats. This insight suggests that tailored treatments specifically targeting these differences could be specially effective in treating stress-related issues.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

