The regulated cell death at the maternal-fetal interface: beneficial or detrimental?
- PMID: 38409106
- PMCID: PMC10897449
- DOI: 10.1038/s41420-024-01867-x
The regulated cell death at the maternal-fetal interface: beneficial or detrimental?
Abstract
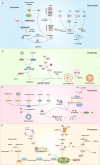
Regulated cell death (RCD) plays a fundamental role in placental development and tissue homeostasis. Placental development relies upon effective implantation and invasion of the maternal decidua by the trophoblast and an immune tolerant environment maintained by various cells at the maternal-fetal interface. Although cell death in the placenta can affect fetal development and even cause pregnancy-related diseases, accumulating evidence has revealed that several regulated cell death were found at the maternal-fetal interface under physiological or pathological conditions, the exact types of cell death and the precise molecular mechanisms remain elusive. In this review, we summarized the apoptosis, necroptosis and autophagy play both promoting and inhibiting roles in the differentiation, invasion of trophoblast, remodeling of the uterine spiral artery and decidualization, whereas ferroptosis and pyroptosis have adverse effects. RCD serves as a mode of communication between different cells to better maintain the maternal-fetal interface microenvironment. Maintaining the balance of RCD at the maternal-fetal interface is of utmost importance for the development of the placenta, establishment of an immune microenvironment, and prevention of pregnancy disorders. In addition, we also revealed an association between abnormal expression of key molecules in different types of RCD and pregnancy-related diseases, which may yield significant insights into the pathogenesis and treatment of pregnancy-related complications.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research.J Hematol Oncol. 2022 Dec 8;15(1):174. doi: 10.1186/s13045-022-01392-3. J Hematol Oncol. 2022. PMID: 36482419 Free PMC article. Review.
-
Periconceptional exposure to lopinavir, but not darunavir, impairs decidualization: a potential mechanism leading to poor birth outcomes in HIV-positive pregnancies.Hum Reprod. 2020 Aug 1;35(8):1781-1796. doi: 10.1093/humrep/deaa151. Hum Reprod. 2020. PMID: 32712670 Free PMC article.
-
Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia.Am J Obstet Gynecol. 2022 Feb;226(2S):S895-S906. doi: 10.1016/j.ajog.2020.09.026. Epub 2020 Sep 21. Am J Obstet Gynecol. 2022. PMID: 32971013 Review.
-
An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array.Am J Obstet Gynecol. 2022 Feb;226(2S):S963-S972. doi: 10.1016/j.ajog.2020.10.023. Epub 2021 Mar 9. Am J Obstet Gynecol. 2022. PMID: 33712272 Review.
-
The function of decidua natural killer cells in physiology and pathology of pregnancy.Am J Reprod Immunol. 2023 Sep;90(3):e13755. doi: 10.1111/aji.13755. Am J Reprod Immunol. 2023. PMID: 37641369 Review.
Cited by
-
Alloimmune Causes of Recurrent Pregnancy Loss: Cellular Mechanisms and Overview of Therapeutic Approaches.Medicina (Kaunas). 2024 Nov 19;60(11):1896. doi: 10.3390/medicina60111896. Medicina (Kaunas). 2024. PMID: 39597081 Free PMC article. Review.
-
Genomic Regions Associated with Spontaneous Abortion in Holstein Heifers.Genes (Basel). 2024 Nov 22;15(12):1498. doi: 10.3390/genes15121498. Genes (Basel). 2024. PMID: 39766766 Free PMC article.
-
Pyroptosis as a therapeutic target in preeclampsia: current research and future directions.Front Immunol. 2025 Jun 25;16:1622550. doi: 10.3389/fimmu.2025.1622550. eCollection 2025. Front Immunol. 2025. PMID: 40636112 Free PMC article. Review.
-
Impact of Ionizing Radiation Exposure on Placental Function and Implications for Fetal Programming.Int J Mol Sci. 2024 Sep 12;25(18):9862. doi: 10.3390/ijms25189862. Int J Mol Sci. 2024. PMID: 39337351 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

