Engineering self-deliverable ribonucleoproteins for genome editing in the brain
- PMID: 38409124
- PMCID: PMC10897210
- DOI: 10.1038/s41467-024-45998-2
Engineering self-deliverable ribonucleoproteins for genome editing in the brain
Erratum in
-
Author Correction: Engineering self-deliverable ribonucleoproteins for genome editing in the brain.Nat Commun. 2024 May 1;15(1):3695. doi: 10.1038/s41467-024-48087-6. Nat Commun. 2024. PMID: 38693162 Free PMC article. No abstract available.
Abstract
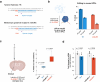
The delivery of CRISPR ribonucleoproteins (RNPs) for genome editing in vitro and in vivo has important advantages over other delivery methods, including reduced off-target and immunogenic effects. However, effective delivery of RNPs remains challenging in certain cell types due to low efficiency and cell toxicity. To address these issues, we engineer self-deliverable RNPs that can promote efficient cellular uptake and carry out robust genome editing without the need for helper materials or biomolecules. Screening of cell-penetrating peptides (CPPs) fused to CRISPR-Cas9 protein identifies potent constructs capable of efficient genome editing of neural progenitor cells. Further engineering of these fusion proteins establishes a C-terminal Cas9 fusion with three copies of A22p, a peptide derived from human semaphorin-3a, that exhibits substantially improved editing efficacy compared to other constructs. We find that self-deliverable Cas9 RNPs generate robust genome edits in clinically relevant genes when injected directly into the mouse striatum. Overall, self-deliverable Cas9 proteins provide a facile and effective platform for genome editing in vitro and in vivo.
© 2024. The Author(s).
Conflict of interest statement
The Regents of the University of California have filed patent applications for self-deliverable CRISPR RNPs under WO 2024/011176, where J.A.D. and K.C. are inventors. J.A.D. is a cofounder of Caribou Biosciences, Editas Medicine, Scribe Therapeutics, Intellia Therapeutics, and Mammoth Biosciences. J.A.D. is a scientific advisory board member or consultant for Vertex, Caribou Biosciences, Intellia Therapeutics, Scribe Therapeutics, Mammoth Biosciences, Algen Biotechnologies, Felix Biosciences, The Column Group, and Inari. J.A.D. is Chief Science Advisor to Sixth Street, a Director at Altos, Johnson & Johnson and Tempus, and she has research projects sponsored by Apple Tree Partners and Roche. The remaining authors declare no competing interests.
Figures




Update of
-
Engineering self-deliverable ribonucleoproteins for genome editing in the brain.bioRxiv [Preprint]. 2023 Nov 15:2023.11.15.567251. doi: 10.1101/2023.11.15.567251. bioRxiv. 2023. Update in: Nat Commun. 2024 Feb 26;15(1):1727. doi: 10.1038/s41467-024-45998-2. PMID: 38014180 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

