Novel mixed heterovalent (Mo/Co)Ox-zerovalent Cu system as bi-functional electrocatalyst for overall water splitting
- PMID: 38409208
- PMCID: PMC10897199
- DOI: 10.1038/s41598-024-54934-9
Novel mixed heterovalent (Mo/Co)Ox-zerovalent Cu system as bi-functional electrocatalyst for overall water splitting
Abstract
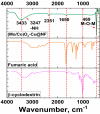
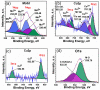
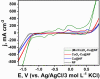
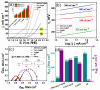
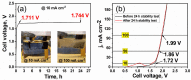
A novel hybrid ternary metallic electrocatalyst of amorphous Mo/Co oxides and crystallized Cu metal was deposited over Ni foam using a one-pot, simple, and scalable solvothermal technique. The chemical structure of the prepared ternary electrocatalyst was systematically characterized and confirmed via XRD, FTIR, EDS, and XPS analysis techniques. FESEM images of (Mo/Co)Ox-Cu@NF display the formation of 3D hierarchical structure with a particle size range of 3-5 µm. The developed (Mo/Co)Ox-Cu@NF ternary electrocatalyst exhibits the maximum activity with 188 mV and 410 mV overpotentials at 50 mA cm-2 for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), respectively. Electrochemical impedance spectroscopy (EIS) results for the (Mo/Co)Ox-Cu@NF sample demonstrate the minimum charge transfer resistance (Rct) and maximum constant phase element (CPE) values. A two-electrode cell based on the ternary electrocatalyst just needs a voltage of about 1.86 V at 50 mA cm-2 for overall water splitting (OWS). The electrocatalyst shows satisfactory durability during the OWS for 24 h at 10 mA cm-2 with an increase of only 33 mV in the cell potential.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
In Situ Grown Mn(II) MOF upon Nickel Foam Acts as a Robust Self-Supporting Bifunctional Electrode for Overall Water Splitting: A Bimetallic Synergistic Collaboration Strategy.ACS Appl Mater Interfaces. 2022 Jul 6;14(26):29722-29734. doi: 10.1021/acsami.2c04304. Epub 2022 Jun 23. ACS Appl Mater Interfaces. 2022. PMID: 35735143
-
Co/Cu-modified NiO film grown on nickel foam as a highly active and stable electrocatalyst for overall water splitting.Dalton Trans. 2020 Feb 14;49(6):1776-1784. doi: 10.1039/c9dt04771a. Epub 2020 Jan 22. Dalton Trans. 2020. PMID: 31967134
-
Non-3d Metal Modulation of a 2D Ni-Co Heterostructure Array as Multifunctional Electrocatalyst for Portable Overall Water Splitting.Small. 2020 Mar;16(10):e1906775. doi: 10.1002/smll.201906775. Epub 2020 Jan 29. Small. 2020. PMID: 31995284
-
Designing In Situ Grown Ternary Oxide/2D Ni-BDC MOF Nanocomposites on Nickel Foam as Efficient Electrocatalysts for Electrochemical Water Splitting.ACS Mater Au. 2022 Dec 28;3(2):143-163. doi: 10.1021/acsmaterialsau.2c00073. eCollection 2023 Mar 8. ACS Mater Au. 2022. PMID: 38089730 Free PMC article.
-
N, P co-doped Ni/Mo-based multicomponent electrocatalysts in situ decorated on Ni foam for overall water splitting.J Colloid Interface Sci. 2023 Sep;645:895-905. doi: 10.1016/j.jcis.2023.04.166. Epub 2023 May 5. J Colloid Interface Sci. 2023. PMID: 37178566
Cited by
-
Unveiling Synergistic Effectiveness of Strategically Designed Cobalt Clusters for Efficient Water Electrolysis.ACS Catal. 2025 Jan 27;15(3):2472-2483. doi: 10.1021/acscatal.4c06466. eCollection 2025 Feb 7. ACS Catal. 2025. PMID: 40463929 Free PMC article.
References
-
- Khan I, Han L, Khan H, Kim Oanh LT. Analyzing renewable and nonrenewable energy sources for environmental quality: Dynamic investigation in developing countries. Math. Probl. Eng. 2021;2021:1–12.
-
- Ang T-Z, et al. A comprehensive study of renewable energy sources: Classifications, challenges and suggestions. Energy Strategy Rev. 2022;43:100939. doi: 10.1016/j.esr.2022.100939. - DOI
-
- Yun S, Zhang Y, Xu Q, Liu J, Qin Y. Recent advance in new-generation integrated devices for energy harvesting and storage. Nano Energy. 2019;60:600–619. doi: 10.1016/j.nanoen.2019.03.074. - DOI
-
- Shenouda AY, Sanad MMS. Synthesis, characterization and electrochemical performance of $$\hbox {Li}_{2}\hbox {Ni}_{x}\hbox {Fe}_{1-x}\hbox {SiO}_{4}$$ Li 2 Ni x Fe 1–x SiO 4 cathode materials for lithium ion batteries. Bull. Mater. Sci. 2017;40:1055–1060. doi: 10.1007/s12034-017-1449-2. - DOI
-
- Sanad MMS, Azab AA, Taha TA. Inducing lattice defects in calcium ferrite anode materials for improved electrochemical performance in lithium-ion batteries. Ceram. Int. 2022;48:12537–12548. doi: 10.1016/j.ceramint.2022.01.121. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

