Deciphering the mechanism of glutaredoxin-catalyzed roGFP2 redox sensing reveals a ternary complex with glutathione for protein disulfide reduction
- PMID: 38409212
- PMCID: PMC10897161
- DOI: 10.1038/s41467-024-45808-9
Deciphering the mechanism of glutaredoxin-catalyzed roGFP2 redox sensing reveals a ternary complex with glutathione for protein disulfide reduction
Abstract
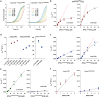
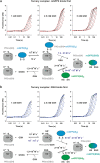
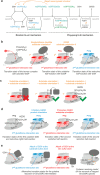
Glutaredoxins catalyze the reduction of disulfides and are key players in redox metabolism and regulation. While important insights were gained regarding the reduction of glutathione disulfide substrates, the mechanism of non-glutathione disulfide reduction remains highly debated. Here we determined the rate constants for the individual redox reactions between PfGrx, a model glutaredoxin from Plasmodium falciparum, and redox-sensitive green fluorescent protein 2 (roGFP2), a model substrate and versatile tool for intracellular redox measurements. We show that the PfGrx-catalyzed oxidation of roGFP2 occurs via a monothiol mechanism and is up to three orders of magnitude faster when roGFP2 and PfGrx are fused. The oxidation kinetics of roGFP2-PfGrx fusion constructs reflect at physiological GSSG concentrations the glutathionylation kinetics of the glutaredoxin moiety, thus allowing intracellular structure-function analysis. Reduction of the roGFP2 disulfide occurs via a monothiol mechanism and involves a ternary complex with GSH and PfGrx. Our study provides the mechanistic basis for understanding roGFP2 redox sensing and challenges previous mechanisms for protein disulfide reduction.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE.J Biol Chem. 2004 Nov 12;279(46):47939-51. doi: 10.1074/jbc.M408011200. Epub 2004 Aug 30. J Biol Chem. 2004. PMID: 15347644
-
An intracellular assay for activity screening and characterization of glutathione-dependent oxidoreductases.Free Radic Biol Med. 2021 Aug 20;172:340-349. doi: 10.1016/j.freeradbiomed.2021.06.016. Epub 2021 Jun 17. Free Radic Biol Med. 2021. PMID: 34146665
-
Quantification of Redox-Sensitive GFP Cysteine Redox State via Gel-Based Read-Out.Methods Mol Biol. 2023;2564:259-268. doi: 10.1007/978-1-0716-2667-2_13. Methods Mol Biol. 2023. PMID: 36107347
-
Molecular Mechanisms of Glutaredoxin Enzymes: Versatile Hubs for Thiol-Disulfide Exchange between Protein Thiols and Glutathione.J Mol Biol. 2019 Jan 18;431(2):158-177. doi: 10.1016/j.jmb.2018.12.006. Epub 2018 Dec 12. J Mol Biol. 2019. PMID: 30552876 Review.
-
Glutaredoxins in thiol/disulfide exchange.Antioxid Redox Signal. 2013 May 1;18(13):1654-65. doi: 10.1089/ars.2012.5007. Epub 2012 Dec 21. Antioxid Redox Signal. 2013. PMID: 23231445 Review.
Cited by
-
Post-Translational Modifications to Cysteine Residues in Plant Proteins and Their Impact on the Regulation of Metabolism and Signal Transduction.Int J Mol Sci. 2024 Sep 12;25(18):9845. doi: 10.3390/ijms25189845. Int J Mol Sci. 2024. PMID: 39337338 Free PMC article. Review.
-
The dithiol mechanism of class I glutaredoxins promotes specificity for glutathione as a reducing agent.Redox Biol. 2024 Dec;78:103410. doi: 10.1016/j.redox.2024.103410. Epub 2024 Oct 24. Redox Biol. 2024. PMID: 39488995 Free PMC article.
-
Fusion of a bacterial cysteine desulfurase to redox-sensitive green fluorescent protein produces a highly sensitive cysteine biosensor for monitoring changes in intracellular cysteine.Redox Biol. 2025 Sep;85:103785. doi: 10.1016/j.redox.2025.103785. Epub 2025 Jul 23. Redox Biol. 2025. PMID: 40720924 Free PMC article.
-
In situ decorated pd NPs on Triazin-encapsulated Fe3O4/SiO2-NH2 as magnetic catalyst for the synthesis of diaryl ethers and oxidation of sulfides.Sci Rep. 2024 Oct 25;14(1):25261. doi: 10.1038/s41598-024-75681-x. Sci Rep. 2024. PMID: 39448720 Free PMC article.
-
A Redox-Sensitive Green Fluorescent Protein (roGFP) Sensing Strategy for Dynamic Analysis of Metal Oxide Nanoparticle-Induced Oxidative Stress.ACS Omega. 2025 Apr 9;10(15):15587-15597. doi: 10.1021/acsomega.5c00774. eCollection 2025 Apr 22. ACS Omega. 2025. PMID: 40290938 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

