Cellular transfection using rapid decrease in hydrostatic pressure
- PMID: 38409237
- PMCID: PMC10897145
- DOI: 10.1038/s41598-024-54463-5
Cellular transfection using rapid decrease in hydrostatic pressure
Abstract
Of all methods exercised in modern molecular biology, modification of cellular properties through the introduction or removal of nucleic acids is one of the most fundamental. As such, several methods have arisen to promote this process; these include the condensation of nucleic acids with calcium, polyethylenimine or modified lipids, electroporation, viral production, biolistics, and microinjection. An ideal transfection method would be (1) low cost, (2) exhibit high levels of biological safety, (3) offer improved efficacy over existing methods, (4) lack requirements for ongoing consumables, (5) work efficiently at any scale, (6) work efficiently on cells that are difficult to transfect by other methods, and (7) be capable of utilizing the widest array of existing genetic resources to facilitate its utility in research, biotechnical and clinical settings. To address such issues, we describe here Pressure-jump-poration (PJP), a method using rapid depressurization to transfect even difficult to modify primary cell types such as embryonic stem cells. The results demonstrate that PJP can be used to introduce an array of genetic modifiers in a safe, sterile manner. Finally, PJP-induced transfection in primary versus transformed cells reveals a surprising dichotomy between these classes which may provide further insight into the process of cellular transformation.
© 2024. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures

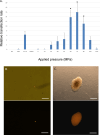
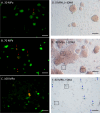
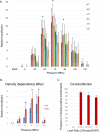
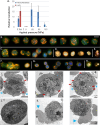

References
-
- Hite B. H. The effect of pressure in the preservation of milk. Bull W. Va. Univ Agric. Experim Stn. 1–67 (1899).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

