NLRP3 inflammasome activation and NETosis positively regulate each other and exacerbate proinflammatory responses: implications of NETosis inhibition for acne skin inflammation treatment
- PMID: 38409251
- PMCID: PMC11061142
- DOI: 10.1038/s41423-024-01137-x
NLRP3 inflammasome activation and NETosis positively regulate each other and exacerbate proinflammatory responses: implications of NETosis inhibition for acne skin inflammation treatment
Abstract
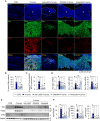
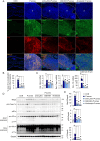
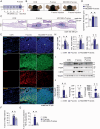
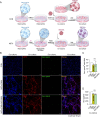
Inflammasomes are multiprotein complexes involved in the host immune response to pathogen infections. Thus, inflammasomes participate in many conditions, such as acne. Recently, it was shown that NETosis, a type of neutrophil cell death, is induced by bacterial infection and is involved in inflammatory diseases such as delayed wound healing in patients with diabetes. However, the relationship between inflammasomes and NETosis in the pathogenesis of inflammatory diseases has not been well studied. In this study, we determined whether NETosis is induced in P. acnes-induced skin inflammation and whether activation of the nucleotide-binding domain, leucine-rich family, and pyrin domain-containing-3 (NLRP3) inflammasome is one of the key factors involved in NETosis induction in a mouse model of acne skin inflammation. We found that NETosis was induced in P. acnes-induced skin inflammation in mice and that inhibition of NETosis ameliorated P. acnes-induced skin inflammation. In addition, our results demonstrated that inhibiting inflammasome activation could suppress NETosis induction in mouse skin. These results indicate that inflammasomes and NETosis can interact with each other to induce P. acnes-induced skin inflammation and suggest that targeting NETosis could be a potential treatment for inflammasome-mediated diseases as well as NETosis-related diseases.
Keywords: P. acnes; NETosis; NLRP3 inflammasome; Skin inflammation.
© 2024. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Quanico J, Gimeno J-P, Nadal-Wollbold F, Casas C, Alvarez-Georges S, Redoules D, et al. Proteomic and transcriptomic investigation of acne vulgaris microcystic and papular lesions: Insights in the understanding of its pathophysiology. Biochim Biophys Acta Gen Subj. 2017;1861:652–63. doi: 10.1016/j.bbagen.2016.10.021. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

