The astrocyte-produced growth factor HB-EGF limits autoimmune CNS pathology
- PMID: 38409259
- PMCID: PMC10907300
- DOI: 10.1038/s41590-024-01756-6
The astrocyte-produced growth factor HB-EGF limits autoimmune CNS pathology
Abstract
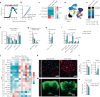
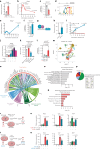
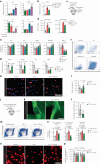
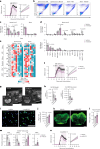
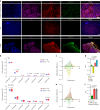
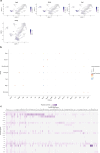
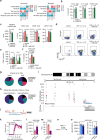
Central nervous system (CNS)-resident cells such as microglia, oligodendrocytes and astrocytes are gaining increasing attention in respect to their contribution to CNS pathologies including multiple sclerosis (MS). Several studies have demonstrated the involvement of pro-inflammatory glial subsets in the pathogenesis and propagation of inflammatory events in MS and its animal models. However, it has only recently become clear that the underlying heterogeneity of astrocytes and microglia can not only drive inflammation, but also lead to its resolution through direct and indirect mechanisms. Failure of these tissue-protective mechanisms may potentiate disease and increase the risk of conversion to progressive stages of MS, for which currently available therapies are limited. Using proteomic analyses of cerebrospinal fluid specimens from patients with MS in combination with experimental studies, we here identify Heparin-binding EGF-like growth factor (HB-EGF) as a central mediator of tissue-protective and anti-inflammatory effects important for the recovery from acute inflammatory lesions in CNS autoimmunity. Hypoxic conditions drive the rapid upregulation of HB-EGF by astrocytes during early CNS inflammation, while pro-inflammatory conditions suppress trophic HB-EGF signaling through epigenetic modifications. Finally, we demonstrate both anti-inflammatory and tissue-protective effects of HB-EGF in a broad variety of cell types in vitro and use intranasal administration of HB-EGF in acute and post-acute stages of autoimmune neuroinflammation to attenuate disease in a preclinical mouse model of MS. Altogether, we identify astrocyte-derived HB-EGF and its epigenetic regulation as a modulator of autoimmune CNS inflammation and potential therapeutic target in MS.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

