Structure-activity characteristics of phenylalanine analogs selectively transported by L-type amino acid transporter 1 (LAT1)
- PMID: 38409393
- PMCID: PMC10897196
- DOI: 10.1038/s41598-024-55252-w
Structure-activity characteristics of phenylalanine analogs selectively transported by L-type amino acid transporter 1 (LAT1)
Abstract
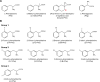
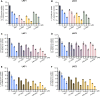
L-type amino acid transporter 1 (LAT1) is a transmembrane protein responsible for transporting large neutral amino acids. While numerous LAT1-targeted compound delivery for the brain and tumors have been investigated, their LAT1 selectivity often remains ambiguous despite high LAT1 affinity. This study assessed the LAT1 selectivity of phenylalanine (Phe) analogs, focusing on their structure-activity characteristics. We discovered that 2-iodo-L-phenylalanine (2-I-Phe), with an iodine substituent at position 2 in the benzene ring, markedly improves LAT1 affinity and selectivity compared to parent amino acid Phe, albeit at the cost of reduced transport velocity. L-Phenylglycine (Phg), one carbon shorter than Phe, was found to be a substrate for LAT1 with a lower affinity, exhibiting a low level of selectivity for LAT1 equivalent to Phe. Notably, (R)-2-amino-1,2,3,4-tetrahydro-2-naphthoic acid (bicyclic-Phe), with an α-methylene moiety akin to the α-methyl group in α-methyl-L-phenylalanine (α-methyl-Phe), a known LAT1-selective compound, showed similar LAT1 transport maximal velocity to α-methyl-Phe, but with higher LAT1 affinity and selectivity. In vivo studies revealed tumor-specific accumulation of bicyclic-Phe, underscoring the importance of LAT1-selectivity in targeted delivery. These findings emphasize the potential of bicyclic-Phe as a promising LAT1-selective component, providing a basis for the development of LAT1-targeting compounds based on its structural framework.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
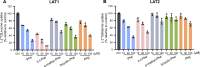



References
-
- Ashique S, Sandhu NK, Chawla V, Chawla PA. Targeted drug delivery: Trends and perspectives. Curr. Drug Deliv. 2021;18:1435–1455. - PubMed
-
- Sai Y, Tsuji A. Transporter-mediated drug delivery: Recent progress and experimental approaches. Drug Discov. Today. 2004;9:712–720. - PubMed
-
- Kanai Y. Amino acid transporter LAT1 (SLC7A5) as a molecular target for cancer diagnosis and therapeutics. Pharmacol. Ther. 2022;230:107964. - PubMed
MeSH terms
Substances
Grants and funding
- [JP19cm0106151]/Japan Agency for Medical Research and Development Project for Cancer Research and Therapeutic Evolution
- [JP20cm0106151]/Japan Agency for Medical Research and Development Project for Cancer Research and Therapeutic Evolution
- [JP21cm0106151]/Japan Agency for Medical Research and Development Project for Cancer Research and Therapeutic Evolution
- [JP23ama221121]/Japan Agency for Medical Research and Development Project for Cancer Research and Therapeutic Evolution
- [19H03407]/Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research
LinkOut - more resources
Full Text Sources
Research Materials

