Visual salience is affected in participants with schizophrenia during free-viewing
- PMID: 38409435
- PMCID: PMC10897421
- DOI: 10.1038/s41598-024-55359-0
Visual salience is affected in participants with schizophrenia during free-viewing
Abstract
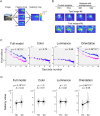
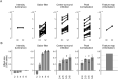
Abnormalities in visual exploration affect the daily lives of patients with schizophrenia. For example, scanpath length during free-viewing is shorter in schizophrenia. However, its origin and its relevance to symptoms are unknown. Here we investigate the possibility that abnormalities in eye movements result from abnormalities in visual or visuo-cognitive processing. More specifically, we examined whether such abnormalities reflect visual salience in schizophrenia. Eye movements of 82 patients and 252 healthy individuals viewing natural and/or complex images were examined using saliency maps for static images to determine the contributions of low-level visual features to salience-guided eye movements. The results showed that the mean value for orientation salience at the gazes of the participants with schizophrenia were higher than that of the healthy control subjects. Further analyses revealed that orientation salience defined by the L + M channel of the DKL color space is specifically affected in schizophrenia, suggesting abnormalities in the magnocellular visual pathway. By looking into the computational stages of the visual salience, we found that the difference between schizophrenia and healthy control emerges at the earlier stage, suggesting functional decline in early visual processing. These results suggest that visual salience is affected in schizophrenia, thereby expanding the concept of the aberrant salience hypothesis of psychosis to the visual domain.
Keywords: Aberrant salience hypothesis; Saccades; Salience.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

