Understanding the Unfolded Protein Response (UPR) Pathway: Insights into Neuropsychiatric Disorders and Therapeutic Potentials
- PMID: 38410073
- PMCID: PMC10902702
- DOI: 10.4062/biomolther.2023.181
Understanding the Unfolded Protein Response (UPR) Pathway: Insights into Neuropsychiatric Disorders and Therapeutic Potentials
Abstract
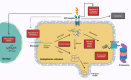
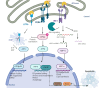
The Unfolded Protein Response (UPR) serves as a critical cellular mechanism dedicated to maintaining protein homeostasis, primarily within the endoplasmic reticulum (ER). This pathway diligently responds to a variety of intracellular indicators of ER stress with the objective of reinstating balance by diminishing the accumulation of unfolded proteins, amplifying the ER's folding capacity, and eliminating slow-folding proteins. Prolonged ER stress and UPR irregularities have been linked to a range of neuropsychiatric disorders, including major depressive disorder, bipolar disorder, and schizophrenia. This review offers a comprehensive overview of the UPR pathway, delineating its activation mechanisms and its role in the pathophysiology of neuropsychiatric disorders. It highlights the intricate interplay within the UPR and its profound influence on brain function, synaptic perturbations, and neural developmental processes. Additionally, it explores evolving therapeutic strategies targeting the UPR within the context of these disorders, underscoring the necessity for precision and further research to effective treatments. The research findings presented in this work underscore the promising potential of UPR-focused therapeutic approaches to address the complex landscape of neuropsychiatric disorders, giving rise to optimism for improving outcomes for individuals facing these complex conditions.
Keywords: Endoplasmic reticulum (ER) stress; Neuropsychiatric disorders; Unfolded protein response.
Figures
Similar articles
-
Endoplasmic Reticulum Homeostasis and Stress Responses in Caenorhabditis elegans.Prog Mol Subcell Biol. 2021;59:279-303. doi: 10.1007/978-3-030-67696-4_13. Prog Mol Subcell Biol. 2021. PMID: 34050871
-
The Role of Endoplasmic Reticulum Stress Response in Pollen Development and Heat Stress Tolerance.Front Plant Sci. 2021 Apr 14;12:661062. doi: 10.3389/fpls.2021.661062. eCollection 2021. Front Plant Sci. 2021. PMID: 33936150 Free PMC article. Review.
-
Proteostasis In The Endoplasmic Reticulum: Road to Cure.Cancers (Basel). 2019 Nov 14;11(11):1793. doi: 10.3390/cancers11111793. Cancers (Basel). 2019. PMID: 31739582 Free PMC article. Review.
-
Endoplasmic reticulum stress: A master regulator of metabolic syndrome.Eur J Pharmacol. 2019 Oct 5;860:172553. doi: 10.1016/j.ejphar.2019.172553. Epub 2019 Jul 17. Eur J Pharmacol. 2019. PMID: 31325433 Review.
-
A small molecule UPR modulator for diabetes identified by high throughput screening.Acta Pharm Sin B. 2021 Dec;11(12):3983-3993. doi: 10.1016/j.apsb.2021.05.018. Epub 2021 Jun 16. Acta Pharm Sin B. 2021. PMID: 35024320 Free PMC article.
Cited by
-
Dissecting Schizophrenia Biology Using Pleiotropy with Cognitive Genomics.medRxiv [Preprint]. 2024 Apr 16:2024.04.16.24305885. doi: 10.1101/2024.04.16.24305885. medRxiv. 2024. Update in: Biol Psychiatry. 2025 Feb 22:S0006-3223(25)00989-8. doi: 10.1016/j.biopsych.2025.02.890. PMID: 38699340 Free PMC article. Updated. Preprint.
-
The Interplay Between Endoplasmic Reticulum Stress and Ferroptosis in Neurological Diseases.Neurochem Res. 2025 Feb 10;50(2):99. doi: 10.1007/s11064-025-04348-4. Neurochem Res. 2025. PMID: 39928173 Review.
-
Emerging and Promising Keywords in Biomolecules and Therapeutics for 21st Century Diseases.Biomol Ther (Seoul). 2025 Jan 1;33(1):1-4. doi: 10.4062/biomolther.2024.007. Epub 2024 Dec 31. Biomol Ther (Seoul). 2025. PMID: 39725476 Free PMC article.
-
Dissecting Schizophrenia Biology Using Pleiotropy With Cognitive Genomics.Biol Psychiatry. 2025 Feb 22:S0006-3223(25)00989-8. doi: 10.1016/j.biopsych.2025.02.890. Online ahead of print. Biol Psychiatry. 2025. PMID: 39993652
-
Sigma-1 Receptor Activation by Fluvoxamine Ameliorates ER Stress, Synaptic Dysfunction and Behavioral Deficits in a Ketamine Model of Schizophrenia.J Neuroimmune Pharmacol. 2025 Jul 25;20(1):76. doi: 10.1007/s11481-025-10231-4. J Neuroimmune Pharmacol. 2025. PMID: 40711497 Free PMC article.
References
-
- Bengesser S. A., Reininghaus E. Z., Lackner N., Birner A., Fellendorf F. T., Platzer M., Kainzbauer N., Tropper B., Hormanseder C., Queissner R., Kapfhammer H. P., Wallner-Liebmann S. J., Fuchs R., Petek E., Windpassinger C., Schnalzenberger M., Reininghaus B., Evert B., Waha A. Is the molecular clock ticking differently in bipolar disorder? Methylation analysis of the clock gene ARNTL. World J. Biol. Psychiatry. 2018;19:S21–S29. doi: 10.1080/15622975.2016.1231421. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

