Assessment of the current status of real-world pharmacogenomic testing: informed consent, patient education, and related practices
- PMID: 38410134
- PMCID: PMC10895424
- DOI: 10.3389/fphar.2024.1355412
Assessment of the current status of real-world pharmacogenomic testing: informed consent, patient education, and related practices
Abstract
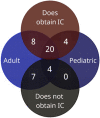
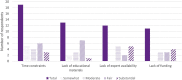
Introduction: The practice of informed consent (IC) for pharmacogenomic testing in clinical settings varies, and there is currently no consensus on which elements of IC to provide to patients. This study aims to assess current IC practices for pharmacogenomic testing. Methods: An online survey was developed and sent to health providers at institutions that offer clinical germline pharmacogenomic testing to assess current IC practices. Results: Forty-six completed surveys representing 43 clinical institutions offering pharmacogenomic testing were received. Thirty-two (74%) respondents obtain IC from patients with variability in elements incorporated. Results revealed that twenty-nine (67%) institutions discuss the benefits, description, and purpose of pharmacogenomic testing with patients. Less commonly discussed elements included methodology and accuracy of testing, and laboratory storage of samples. Discussion: IC practices varied widely among survey respondents. Most respondents desire the establishment of consensus IC recommendations from a trusted pharmacogenomics organization to help address these disparities.
Keywords: clinical implementation; genetic counseling; genetic testing; informed consent; pharmacogenetics; pharmacogenomics.
Copyright © 2024 Pereira, Haidar, Haga, Cisler, Hall, Shukla, Hebbring and Leary.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Accreditation Council for Genetic Counseling (2003). Practice-Based Competencies for Genetic Counselors. McLean, VA, August, 31.
-
- AMA (2013). AMA code of medical ethics’ opinion on informing patients about treatment options. AMA J. Ethics 15 (1), 28. 10.1001/virtualmentor.2013.15.1.coet1-1301 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

