Combining photodynamic therapy and cascade chemotherapy for enhanced tumor cytotoxicity: the role of CTT2P@B nanoparticles
- PMID: 38410166
- PMCID: PMC10895035
- DOI: 10.3389/fbioe.2024.1361966
Combining photodynamic therapy and cascade chemotherapy for enhanced tumor cytotoxicity: the role of CTT2P@B nanoparticles
Abstract
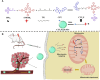
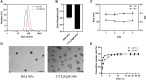
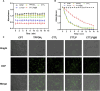
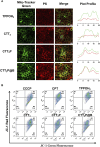
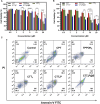
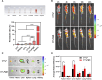
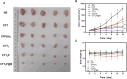
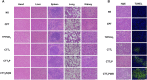
The mitochondria act as the main producers of reactive oxygen species (ROS) within cells. Elevated levels of ROS can activate the mitochondrial apoptotic pathway, leading to cell apoptosis. In this study, we devised a molecular prodrug named CTT2P, demonstrating notable efficacy in facilitating mitochondrial apoptosis. To develop nanomedicine, we enveloped CTT2P within bovine serum albumin (BSA), resulting in the formulation known as CTT2P@B. The molecular prodrug CTT2P is achieved by covalently conjugating mitochondrial targeting triphenylphosphine (PPh3), photosensitizer TPPOH2, ROS-sensitive thioketal (TK), and chemotherapeutic drug camptothecin (CPT). The prodrug, which is chemically bonded, prevents the escape of drugs while they circulate throughout the body, guaranteeing the coordinated dispersion of both medications inside the organism. Additionally, the concurrent integration of targeted photodynamic therapy and cascade chemotherapy synergistically enhances the therapeutic efficacy of pharmaceutical agents. Experimental results indicated that the covalently attached prodrug significantly mitigated CPT cytotoxicity under dark conditions. In contrast, TPPOH2, CTT2, CTT2P, and CTT2P@B nanoparticles exhibited increasing tumor cell-killing effects and suppressed tumor growth when exposed to light at 660 nm with an intensity of 280 mW cm-2. Consequently, this laser-triggered, mitochondria-targeted, combined photodynamic therapy and chemotherapy nano drug delivery system, adept at efficiently promoting mitochondrial apoptosis, presents a promising and innovative approach to cancer treatment.
Keywords: ROS-responsive; bovine serum albumin; camptothecin; mitochondria-targeting; photodynamic therapy.
Copyright © 2024 Wang, Li, Han, Han, Bao, Fan, Sun, Qian, Ma and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
ROS-Responsive Mitochondria-Targeting Blended Nanoparticles: Chemo- and Photodynamic Synergistic Therapy for Lung Cancer with On-Demand Drug Release upon Irradiation with a Single Light Source.Theranostics. 2016 Oct 1;6(13):2352-2366. doi: 10.7150/thno.15433. eCollection 2016. Theranostics. 2016. PMID: 27877240 Free PMC article.
-
Bioactivatable reactive oxygen species-sensitive nanoparticulate system for chemo-photodynamic therapy.Acta Biomater. 2020 May;108:273-284. doi: 10.1016/j.actbio.2020.03.027. Epub 2020 Mar 21. Acta Biomater. 2020. PMID: 32205212
-
ROS-cleavable diselenide nanomedicine for NIR-controlled drug release and on-demand synergistic chemo-photodynamic therapy.Acta Biomater. 2022 Nov;153:442-452. doi: 10.1016/j.actbio.2022.09.061. Epub 2022 Sep 28. Acta Biomater. 2022. PMID: 36179978
-
ATP-triggered mitochondrial cascade reactions for cancer therapy with nanoscale zeolitic imidazole framework-90.Theranostics. 2021 Jun 26;11(16):7869-7878. doi: 10.7150/thno.59593. eCollection 2021. Theranostics. 2021. PMID: 34335969 Free PMC article.
-
Prodrug strategies for targeted therapy triggered by reactive oxygen species.Medchemcomm. 2019 May 8;10(9):1531-1549. doi: 10.1039/c9md00169g. eCollection 2019 Sep 1. Medchemcomm. 2019. PMID: 31673314 Free PMC article. Review.
Cited by
-
Application of photodynamic activation of prodrugs combined with phototherapy in tumor treatment.Mol Cancer. 2025 Jul 21;24(1):200. doi: 10.1186/s12943-025-02404-9. Mol Cancer. 2025. PMID: 40685358 Free PMC article. Review.
References
-
- Agwa M. M., Elmotasem H., Elsayed H., Abdelsattar A. S., Omer A. M., Gebreel D. T., et al. (2023). Carbohydrate ligands-directed active tumor targeting of combinatorial chemotherapy/phototherapy-based nanomedicine: a review. Int. J. Biol. Macromol. 239, 124294. 10.1016/j.ijbiomac.2023.124294 - DOI - PubMed
-
- Chu B., Qu Y., He X., Hao Y., Yang C., Yang Y., et al. (2020). ROS‐Responsive camptothecin prodrug nanoparticles for on‐demand drug release and combination of chemotherapy and photodynamic therapy. Adv. Funct. Mat. 30 (52). 10.1002/adfm.202005918 - DOI
LinkOut - more resources
Full Text Sources

