Can we successfully define and target BRCA-like breast cancers?
- PMID: 38410223
- PMCID: PMC10894354
- DOI: 10.21037/tcr-23-577
Can we successfully define and target BRCA-like breast cancers?
Keywords: BRCA-like; Poly (ADP-ribose) polymerase (PARP); biomarker; homologous recombination deficiency (HRD); veliparib.
Conflict of interest statement
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-577/coif). N.L. discloses receiving funds for participating in Advisory boards with the following: Astra Zeneca, Eli Lilly, Gilead, Merck, Knight Therapeutics, Novartis, Pfizer, Roche, Seagen, and TerSera, and receiving research funds to their institution from the following: Abbvie, Avon Foundation for Women, BC Cancer Foundation, CCS, CIHR, Eli Lilly, Exact Sciences, Gilead, and Michael Smith Health Research. K.A.G. declares that she receives research grants with funds to her institution from AstraZeneca, Pfizer and BMS, and payment or honoraria for Speakers event 2023 from Astra Zeneca. She also participates on the Advisory Board of Eli Lilly, Gilead, Merck, Novartis, Seagen and Testara, Advisory board and DMC of Astra Zeneca and Pfizer, and DMC of City of Hope. The authors have no other conflicts of interest to declare.
Figures
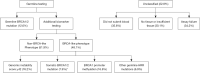
Comment on
-
Cisplatin with veliparib or placebo in metastatic triple-negative breast cancer and BRCA mutation-associated breast cancer (S1416): a randomised, double-blind, placebo-controlled, phase 2 trial.Lancet Oncol. 2023 Feb;24(2):162-174. doi: 10.1016/S1470-2045(22)00739-2. Epub 2023 Jan 6. Lancet Oncol. 2023. PMID: 36623515 Free PMC article. Clinical Trial.
References
Publication types
LinkOut - more resources
Full Text Sources
