Covariates in population pharmacokinetic studies of critically ill adults receiving β-lactam antimicrobials: a systematic review and narrative synthesis
- PMID: 38410250
- PMCID: PMC10895699
- DOI: 10.1093/jacamr/dlae030
Covariates in population pharmacokinetic studies of critically ill adults receiving β-lactam antimicrobials: a systematic review and narrative synthesis
Abstract
Introduction: Population pharmacokinetic studies of β-lactam antimicrobials in critically ill patients derive models that inform their dosing. In non-linear mixed-effects modelling, covariates are often used to improve model fit and explain variability. We aimed to investigate which covariates are most commonly assessed and which are found to be significant, along with global patterns of publication.
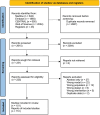
Methods: We conducted a systematic review, searching MEDLINE, Embase, CENTRAL and Web of Science on 01 March 2023, including studies of critically ill adults receiving β-lactam antimicrobials who underwent blood sampling for population pharmacokinetic studies. We extracted and categorized all reported covariates and assessed reporting quality using the ClinPK checklist.
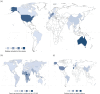
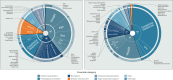
Results: Our search identified 151 studies with 6018 participants. Most studies reported observational cohorts (120 studies, 80%), with the majority conducted in high-income settings (136 studies, 90%). Of the 1083 identified covariate instances, 237 were unique; the most common categories were patient characteristics (n = 404), biomarkers (n = 206) and physiological parameters (n = 163). Only seven distinct commonly reported covariates (CLCR, weight, glomerular filtration rate, diuresis, need for renal replacement, serum albumin and C-reactive protein) were significant more than 20% of the time.
Conclusions: Covariates are most commonly chosen based on biological plausibility, with patient characteristics and biomarkers the most frequently investigated. We developed an openly accessible database of reported covariates to aid investigators with covariate selection when designing population pharmacokinetic studies. Novel covariates, such as sepsis subphenotypes, have not been explored yet, leaving a research gap for future work.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
