Facile approach to N,O,S-heteropentacycles via condensation of sterically crowded 3 H-phenoxazin-3-one with ortho- substituted anilines
- PMID: 38410782
- PMCID: PMC10896220
- DOI: 10.3762/bjoc.20.34
Facile approach to N,O,S-heteropentacycles via condensation of sterically crowded 3 H-phenoxazin-3-one with ortho- substituted anilines
Abstract
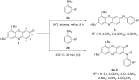
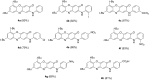
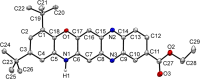
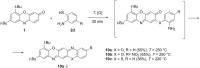
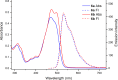
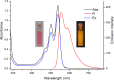
A convenient method for the synthesis of a series of 2-(arylamino)-3H-phenoxazin-3-ones based on the nucleophilic substitution reaction between sterically crowded 3H-phenoxazin-3-one and arylamines performed by short-term heating of the melted reactants at 220-250 °C is described, and the compounds were characterized by means of single-crystal X-ray crystallography, NMR, UV-vis, and IR spectroscopy, as well as cyclic voltammetry. The reaction with o-amino-, o-hydroxy-, and o-mercapto-substituted arylamines widened the scope and provided an access to derivatives of N,O- and N,S-heteropentacyclic quinoxalinophenoxazine, triphenodioxazine and oxazinophenothiazine systems.
Keywords: 3H-phenoxazin-3-one; fluorescence; molecular structure; pentacyclic heterocycles; synthesis.
Copyright © 2024, Ivakhnenko et al.
Figures






References
-
- Diepolder E. Ber Dtsch Chem Ges. 1902;35(3):2816–2822. doi: 10.1002/cber.19020350360. - DOI
-
- Podder N, Mandal S. New J Chem. 2020;44:12793–12805. doi: 10.1039/d0nj02558e. - DOI
-
- Abakumov G A, Druzhkov N O, Kurskii Y A, Abakumova L G, Shavyrin A S, Fukin G K, Poddel’skii A I, Cherkasov V K, Okhlopkova L S. Russ Chem Bull. 2005;54(11):2571–2577. doi: 10.1007/s11172-006-0157-7. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
