Efficacy and Safety of Generic Alendronate for Osteoporosis Treatment
- PMID: 38410814
- PMCID: PMC10895978
- DOI: 10.2147/ORR.S445202
Efficacy and Safety of Generic Alendronate for Osteoporosis Treatment
Abstract
Background: While osteoporosis increases the risk of fragility fractures, bisphosphonate has been proven to increase bone strength and reduce the risk of vertebral and non-vertebral fractures. In addition to its efficacy, substituting the brand with generic medication is a strategy to optimize healthcare expenditures. This study aimed to evaluate the efficacy of generic alendronate treatment and assess potential adverse events in patients with osteoporosis.
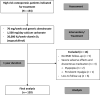
Materials and methods: A retrospective review was conducted on 120 patients who met the indications for osteoporosis treatment, received weekly generic alendronate (70 mg) for >1 year, and underwent evaluation through standard axial dual-energy X-ray absorptiometry (DXA). The outcomes of this study were the percent change in bone mineral density (BMD) at the lumbar spine, femoral neck, and total hip after one year of treatment. The major adverse events occurring during medication that led to the discontinuation of drug administration were documented.
Results: Most patients were female (96.7%) with an average age of 69.0 ± 9.3 years. The percent change in BMD increased at all sites after one year of generic alendronate treatment (lumbar spine: 5.6 ± 13.7, p-value <0.001; femoral neck: 2.3 ± 8.3, p-value = 0.023; total hip: 2.1 ± 6.2, p-value = 0.003), with over 85% of patients experiencing increased or stable BMD. Three patients discontinued the medication due to adverse effects: two had dyspepsia, and one had persistent myalgia.
Conclusion: Generic alendronate may be considered an effective antiresorptive agent for osteoporosis treatment with a low incidence of adverse effects.
Keywords: alendronate; bisphosphonate; bone mineral density; fragility fracture; osteoporosis.
© 2024 Jarusriwanna et al.
Conflict of interest statement
The authors declare no personal or professional conflicts of interest regarding any aspect of this study.
Figures
Similar articles
-
Randomized clinical trial comparing efficacy and safety of brand versus generic alendronate (Bonmax®) for osteoporosis treatment.PLoS One. 2017 Jul 5;12(7):e0180325. doi: 10.1371/journal.pone.0180325. eCollection 2017. PLoS One. 2017. PMID: 28678853 Free PMC article. Clinical Trial.
-
Utilization of DXA Bone Mineral Densitometry in Ontario: An Evidence-Based Analysis.Ont Health Technol Assess Ser. 2006;6(20):1-180. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2006. PMID: 23074491 Free PMC article.
-
Differences in persistence, safety and efficacy of generic and original branded once weekly bisphosphonates in patients with postmenopausal osteoporosis: 1-year results of a retrospective patient chart review analysis.Rheumatol Int. 2009 Dec;30(2):213-21. doi: 10.1007/s00296-009-0940-5. Rheumatol Int. 2009. PMID: 19430791
-
Alendronate for the Treatment of Osteoporosis in Men: A Meta-Analysis of Randomized Controlled Trials.Am J Ther. 2017 Mar/Apr;24(2):e130-e138. doi: 10.1097/MJT.0000000000000446. Am J Ther. 2017. PMID: 27058577 Review.
-
Bone health in childhood and adolescence: an overview on dual-energy X-ray absorptiometry scanning, fracture surveillance and bisphosphonate therapy for low-middle-income countries.Front Endocrinol (Lausanne). 2023 Apr 17;14:1082413. doi: 10.3389/fendo.2023.1082413. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37139332 Free PMC article. Review.
Cited by
-
Atypical Tibial Fracture Following Chronic Bisphosphonate Use: A Case Report and Review of the Literature.Cureus. 2024 Nov 6;16(11):e73165. doi: 10.7759/cureus.73165. eCollection 2024 Nov. Cureus. 2024. PMID: 39650869 Free PMC article.
-
Alendronate for Effective Treatment of Male Osteoporosis: An Insight.Curr Pharm Des. 2025;31(1):26-36. doi: 10.2174/0113816128310838240820065324. Curr Pharm Des. 2025. PMID: 39238374 Review.
References
-
- Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1):136. doi:10.1007/s11657-013-0136-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous