Repetitive sulfur dioxide exposure in mice models post-deployment respiratory syndrome
- PMID: 38410870
- PMCID: PMC11380962
- DOI: 10.1152/ajplung.00239.2023
Repetitive sulfur dioxide exposure in mice models post-deployment respiratory syndrome
Abstract
Soldiers deployed to Iraq and Afghanistan have a higher prevalence of respiratory symptoms than nondeployed military personnel and some have been shown to have a constellation of findings on lung biopsy termed post-deployment respiratory syndrome (PDRS). Since many of the subjects in this cohort reported exposure to sulfur dioxide (SO2), we developed a model of repetitive exposure to SO2 in mice that phenocopies many aspects of PDRS, including adaptive immune activation, airway wall remodeling, and pulmonary vascular (PV) disease. Although abnormalities in small airways were not sufficient to alter lung mechanics, PV remodeling resulted in the development of pulmonary hypertension and reduced exercise tolerance in SO2-exposed mice. SO2 exposure led to increased formation of isolevuglandins (isoLGs) adducts and superoxide dismutase 2 (SOD2) acetylation in endothelial cells, which were attenuated by treatment with the isoLG scavenger 2-hydroxybenzylamine acetate (2-HOBA). In addition, 2-HOBA treatment or Siruin-3 overexpression in a transgenic mouse model prevented vascular remodeling following SO2 exposure. In summary, our results indicate that repetitive SO2 exposure recapitulates many aspects of PDRS and that oxidative stress appears to mediate PV remodeling in this model. Together, these findings provide new insights regarding the critical mechanisms underlying PDRS.NEW & NOTEWORTHY We developed a mice model of "post-deployment respiratory syndrome" (PDRS), a condition in Veterans with unexplained exertional dyspnea. Our model successfully recapitulates many of the pathological and physiological features of the syndrome, revealing involvement of the ROS-isoLGs-Sirt3-SOD2 pathway in pulmonary vasculature pathology. Our study provides additional knowledge about effects and long-term consequences of sulfur dioxide exposure on the respiratory system, serving as a valuable tool for future PDRS research.
Keywords: constrictive bronchiolitis; experimental animal models; oxidative stress; pulmonary hypertension; sulfur dioxide.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



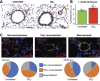
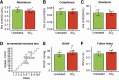

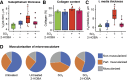

Update of
-
Repetitive Sulfur Dioxide Exposure in Mice Models Post-Deployment Respiratory Syndrome.bioRxiv [Preprint]. 2023 May 18:2023.05.15.540867. doi: 10.1101/2023.05.15.540867. bioRxiv. 2023. Update in: Am J Physiol Lung Cell Mol Physiol. 2024 May 1;326(5):L539-L550. doi: 10.1152/ajplung.00239.2023. PMID: 37292948 Free PMC article. Updated. Preprint.
Similar articles
-
Repetitive Sulfur Dioxide Exposure in Mice Models Post-Deployment Respiratory Syndrome.bioRxiv [Preprint]. 2023 May 18:2023.05.15.540867. doi: 10.1101/2023.05.15.540867. bioRxiv. 2023. Update in: Am J Physiol Lung Cell Mol Physiol. 2024 May 1;326(5):L539-L550. doi: 10.1152/ajplung.00239.2023. PMID: 37292948 Free PMC article. Updated. Preprint.
-
Sulfur Dioxide Protects Against Collagen Accumulation in Pulmonary Artery in Association With Downregulation of the Transforming Growth Factor β1/Smad Pathway in Pulmonary Hypertensive Rats.J Am Heart Assoc. 2016 Oct 17;5(10):e003910. doi: 10.1161/JAHA.116.003910. J Am Heart Assoc. 2016. PMID: 27792648 Free PMC article.
-
Pulmonary vascular disease in Veterans with post-deployment respiratory syndrome.Cardiovasc Pathol. 2024 Jul-Aug;71:107640. doi: 10.1016/j.carpath.2024.107640. Epub 2024 Apr 9. Cardiovasc Pathol. 2024. PMID: 38604505
-
Sulfur dioxide exposure of mice induces peribronchiolar fibrosis-A defining feature of deployment-related constrictive bronchiolitis.PLoS One. 2025 Jan 24;20(1):e0313992. doi: 10.1371/journal.pone.0313992. eCollection 2025. PLoS One. 2025. PMID: 39854594 Free PMC article.
-
Role of Endogenous Sulfur Dioxide in Regulating Vascular Structural Remodeling in Hypertension.Oxid Med Cell Longev. 2016;2016:4529060. doi: 10.1155/2016/4529060. Epub 2016 Sep 18. Oxid Med Cell Longev. 2016. PMID: 27721913 Free PMC article. Review.
Cited by
-
RNA Sequencing Reveals Inflammatory and Metabolic Changes in the Lung and Brain After Carbon Black and Naphthalene Whole Body Inhalation Exposure in a Rodent Model of Military Burn Pit Exposures.Int J Mol Sci. 2025 Jul 26;26(15):7238. doi: 10.3390/ijms26157238. Int J Mol Sci. 2025. PMID: 40806382 Free PMC article.
-
Current understanding of the impact of United States military airborne hazards and burn pit exposures on respiratory health.Part Fibre Toxicol. 2024 Oct 21;21(1):43. doi: 10.1186/s12989-024-00606-5. Part Fibre Toxicol. 2024. PMID: 39434148 Free PMC article. Review.
References
-
- National Academies, Sciences, and Engineering; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. Washington, DC: National Academies Press (US), 2020. - PubMed
-
- Garshick E, Abraham JH, Baird CP, Ciminera P, Downey GP, Falvo MJ, Hart JE, Jackson DA, Jerrett M, Kuschner W, Helmer DA, Jones KD, Krefft SD, Mallon T, Miller RF, Morris MJ, Proctor SP, Redlich CA, Rose CS, Rull RP, Saers J, Schneiderman AI, Smith NL, Yiallouros P, Blanc PD. Respiratory health after military service in Southwest Asia and Afghanistan. An official American Thoracic Society Workshop report. Ann Am Thorac Soc 16: e1–e16, 2019. doi:10.1513/AnnalsATS.201904-344WS. - DOI - PMC - PubMed
-
- Morris MJ, Walter RJ, McCann ET, Sherner JH, Murillo CG, Barber BS, Hunninghake JC, Holley AB. Clinical evaluation of deployed military personnel with chronic respiratory symptoms: study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE) III. Chest 157: 1559–1567, 2020. doi:10.1016/j.chest.2020.01.024. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

