Cellulose-Callose Hydrogels: Computational Exploration of Their Nanostructure and Mechanical Properties
- PMID: 38410888
- PMCID: PMC10934845
- DOI: 10.1021/acs.biomac.3c01396
Cellulose-Callose Hydrogels: Computational Exploration of Their Nanostructure and Mechanical Properties
Abstract
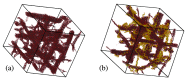
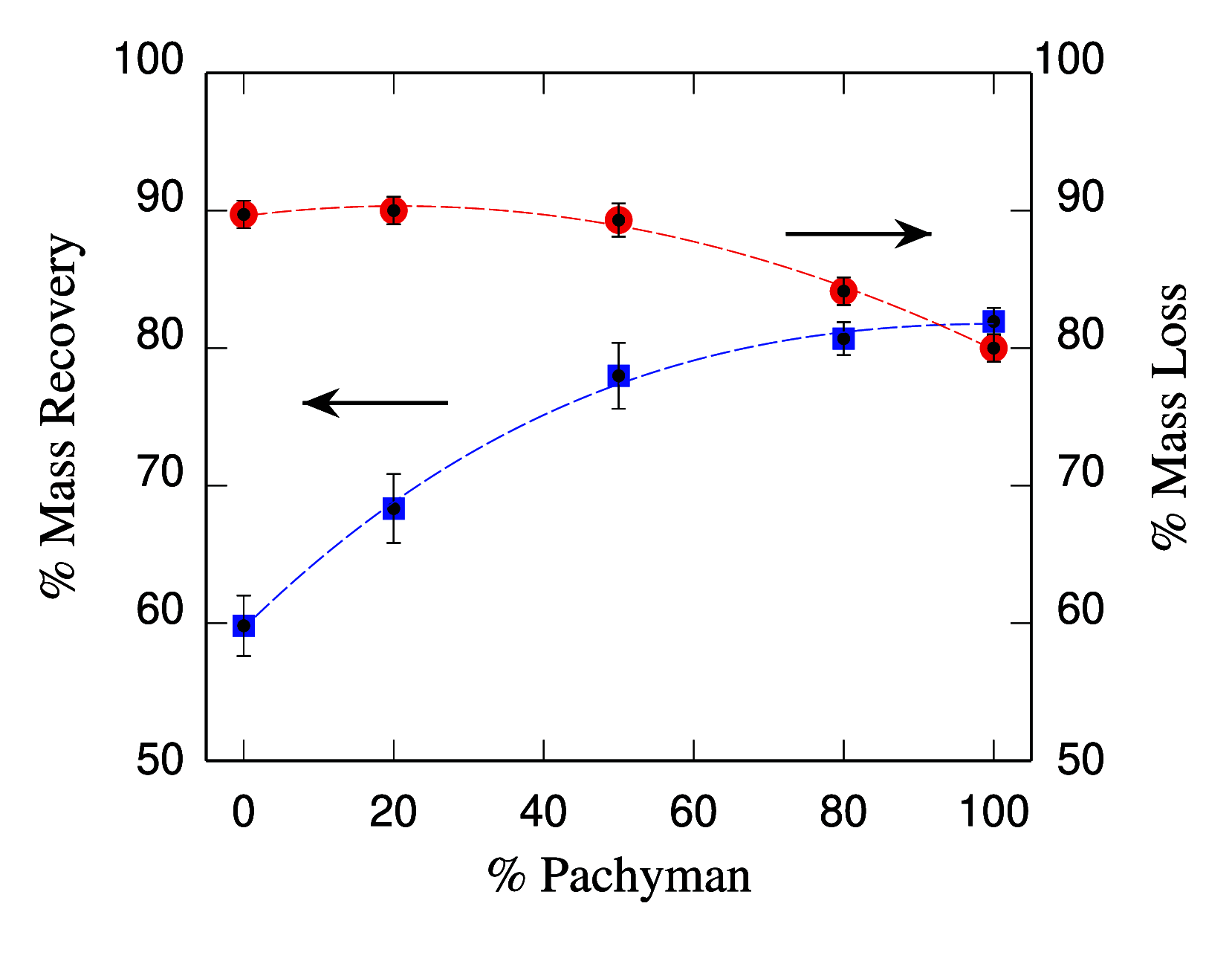
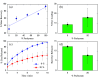
Polysaccharides play a crucial role in virtually all living systems. They also represent the biocompatible and fully sustainable component of a variety of nanoparticles, which are of increasing interest in biomedicine, food processing, cosmetics, and structural reinforcement of polymeric materials. The computational modeling of complex polysaccharide phases will assist in understanding the properties and behavior of all these systems. In this paper, structural, bonding, and mechanical properties of 10 wt % cellulose-callose hydrogels (β-glucans coexisting in plant cell walls) were investigated by atomistic simulations. Systems of this kind have recently been introduced in experiments revealing unexpected interactions between the polysaccharides. Starting from initial configurations inspired by X-ray diffraction data, atomistic models made of ∼1.6 × 106 atoms provide a qualitatively consistent view of these hydrogels, displaying stability, homogeneity, connectivity, and elastic properties beyond those of a liquid suspension. The simulation shows that the relatively homogeneous distribution of saccharide nanofibers and chains in water is not due to the solubility of cellulose and callose, but to the formation of a number of cross-links among the various sample components. The broad distribution of strength and elasticity among the links implies a degree of anharmonicity and irreversible deformation already evident at low external load. Besides the qualitative agreement with experimental observations, the simulation results display also quantitative disagreements in the estimation of elastic coefficients, such as the Young's modulus, that require further investigation. Complementary simulations of dense cellulose-callose mixtures (no hydrogels) highlight the role of callose in smoothing the contact surface of different nanofibers forming larger bundles. Cellulose-callose structures in these systems displayed an enhanced water uptake and delayed dye release when compared to cellulose alone, highlighting potential new applications as drug delivery scaffolds. The simulation trajectories provide a tuning and testing ground for the development of coarse-grained models that are required for the large scale investigation of mechanical properties of cellulose and callose mixtures in a watery environment.
Conflict of interest statement
The authors declare no competing financial interest.
Figures












Similar articles
-
Simulation Study of the Water Ordering Effect of the β-(1,3)-Glucan Callose Biopolymer.Biomacromolecules. 2025 Mar 10;26(3):1748-1760. doi: 10.1021/acs.biomac.4c01524. Epub 2025 Feb 5. Biomacromolecules. 2025. PMID: 39907440 Free PMC article.
-
Interactions between callose and cellulose revealed through the analysis of biopolymer mixtures.Nat Commun. 2018 Oct 31;9(1):4538. doi: 10.1038/s41467-018-06820-y. Nat Commun. 2018. PMID: 30382102 Free PMC article.
-
Poroelastic mechanical effects of hemicelluloses on cellulosic hydrogels under compression.PLoS One. 2015 Mar 20;10(3):e0122132. doi: 10.1371/journal.pone.0122132. eCollection 2015. PLoS One. 2015. PMID: 25794048 Free PMC article.
-
Cellulose and callose synthesis and organization in focus, what's new?Curr Opin Plant Biol. 2016 Dec;34:9-16. doi: 10.1016/j.pbi.2016.07.007. Epub 2016 Jul 29. Curr Opin Plant Biol. 2016. PMID: 27479608 Review.
-
Cellulose Nanofibrils-based Hydrogels for Biomedical Applications: Progresses and Challenges.Curr Med Chem. 2020;27(28):4622-4646. doi: 10.2174/0929867327666200303102859. Curr Med Chem. 2020. PMID: 32124687 Review.
Cited by
-
Simulation Study of the Water Ordering Effect of the β-(1,3)-Glucan Callose Biopolymer.Biomacromolecules. 2025 Mar 10;26(3):1748-1760. doi: 10.1021/acs.biomac.4c01524. Epub 2025 Feb 5. Biomacromolecules. 2025. PMID: 39907440 Free PMC article.
-
Computer-Assisted Strategies as a Tool for Designing Green Monomer-Based Molecularly Imprinted Materials.Int J Mol Sci. 2024 Nov 30;25(23):12912. doi: 10.3390/ijms252312912. Int J Mol Sci. 2024. PMID: 39684622 Free PMC article. Review.
References
-
- Hasan N.; Rahman L.; Kim S.-H.; Cao J.; Arjuna A.; Lallo S.; Jhun B. H.; Yoo J.-W. Recent advances of nanocellulose indrug delivery systems. J. Pharm. Investig. 2020, 50, 553–572. 10.1007/s40005-020-00499-4. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

