RpoS and the bacterial general stress response
- PMID: 38411096
- PMCID: PMC10966952
- DOI: 10.1128/mmbr.00151-22
RpoS and the bacterial general stress response
Abstract
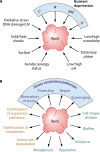
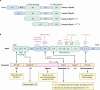
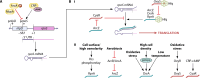
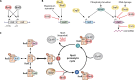
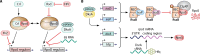
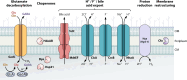
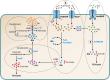
SUMMARYThe general stress response (GSR) is a widespread strategy developed by bacteria to adapt and respond to their changing environments. The GSR is induced by one or multiple simultaneous stresses, as well as during entry into stationary phase and leads to a global response that protects cells against multiple stresses. The alternative sigma factor RpoS is the central GSR regulator in E. coli and conserved in most γ-proteobacteria. In E. coli, RpoS is induced under conditions of nutrient deprivation and other stresses, primarily via the activation of RpoS translation and inhibition of RpoS proteolysis. This review includes recent advances in our understanding of how stresses lead to RpoS induction and a summary of the recent studies attempting to define RpoS-dependent genes and pathways.
Keywords: RssB; small RNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

