Intranasal Epitope-Polymer Vaccine Lodges Resident Memory T Cells Protecting Against Influenza Virus
- PMID: 38411375
- PMCID: PMC11469178
- DOI: 10.1002/adhm.202304188
Intranasal Epitope-Polymer Vaccine Lodges Resident Memory T Cells Protecting Against Influenza Virus
Abstract
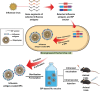
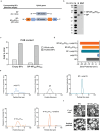
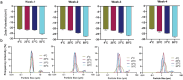
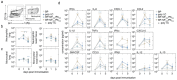
Intranasal vaccines, unlike injectable vaccines, boost immunity along the respiratory tract; this can significantly limit respiratory virus replication and shedding. There remains a need to develop mucosal adjuvants and vaccine delivery systems that are both safe and effective following intranasal administration. Here, biopolymer particles (BP) densely coated with repeats of MHC class I restricted immunodominant epitopes derived from influenza A virus namely NP366, a nucleoprotein-derived epitope and PA224, a polymerase acidic subunit derived epitope, are bioengineered. These BP-NP366/PA224 can be manufactured at a high yield and are obtained at ≈93% purity, exhibiting ambient-temperature stability. Immunological characterization includes comparing systemic and mucosal immune responses mounted following intramuscular or intranasal immunization. Immunization with BP-NP366/PA224 without adjuvant triggers influenza-specific CD8+ T cell priming and memory CD8+ T cell development. Co-delivery with the adjuvant poly(I:C) significantly boosts the size and functionality of the influenza-specific pulmonary resident memory CD8+ T cell pool. Intranasal, but not intramuscular delivery of BP-NP366/PA224 with poly(I:C), provides protection against influenza virus challenge. Overall, the BP approach demonstrates as a suitable antigen formulation for intranasal delivery toward induction of systemic protective T cell responses against influenza virus.
Keywords: influenza; intranasal delivery; polyhydroxybutyrate; resident memory T cells; subunit vaccine; vaccine.
© 2024 The Authors. Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
B.H.A.R. is a cofounder and shareholder of PolyBatics Ltd.
Figures



Similar articles
-
CCR2 Regulates Vaccine-Induced Mucosal T-Cell Memory to Influenza A Virus.J Virol. 2021 Jul 12;95(15):e0053021. doi: 10.1128/JVI.00530-21. Epub 2021 Jul 12. J Virol. 2021. PMID: 33952647 Free PMC article.
-
Effective Respiratory CD8 T-Cell Immunity to Influenza Virus Induced by Intranasal Carbomer-Lecithin-Adjuvanted Non-replicating Vaccines.PLoS Pathog. 2016 Dec 20;12(12):e1006064. doi: 10.1371/journal.ppat.1006064. eCollection 2016 Dec. PLoS Pathog. 2016. PMID: 27997610 Free PMC article.
-
Intranasal administration of a synthetic peptide vaccine encapsulated in liposome together with an anti-CD40 antibody induces protective immunity against influenza A virus in mice.Vaccine. 2002 Aug 19;20(25-26):3123-9. doi: 10.1016/s0264-410x(02)00261-x. Vaccine. 2002. PMID: 12163263
-
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article.
-
Single mucosal vaccination targeting nucleoprotein provides broad protection against two lineages of influenza B virus.Antiviral Res. 2019 Mar;163:19-28. doi: 10.1016/j.antiviral.2019.01.002. Epub 2019 Jan 9. Antiviral Res. 2019. PMID: 30639307
Cited by
-
T-Cell Epitope-Based Vaccines: A Promising Strategy for Prevention of Infectious Diseases.Vaccines (Basel). 2024 Oct 17;12(10):1181. doi: 10.3390/vaccines12101181. Vaccines (Basel). 2024. PMID: 39460347 Free PMC article. Review.
-
Advances in protein subunit vaccines against H1N1/09 influenza.Front Immunol. 2024 Nov 22;15:1499754. doi: 10.3389/fimmu.2024.1499754. eCollection 2024. Front Immunol. 2024. PMID: 39650643 Free PMC article. Review.
-
Memory T Cells in Respiratory Virus Infections: Protective Potential and Persistent Vulnerabilities.Med Sci (Basel). 2025 Apr 29;13(2):48. doi: 10.3390/medsci13020048. Med Sci (Basel). 2025. PMID: 40407543 Free PMC article. Review.
References
-
- Wakim L. M., Smith J., Caminschi I., Lahoud M. H., Villadangos J. A., Mucosal Immunol. 2015, 8, 1060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

