T2WI-based MRI radiomics for the prediction of preoperative extranodal extension and prognosis in resectable rectal cancer
- PMID: 38411722
- PMCID: PMC10899552
- DOI: 10.1186/s13244-024-01625-8
T2WI-based MRI radiomics for the prediction of preoperative extranodal extension and prognosis in resectable rectal cancer
Abstract
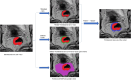
Objective: To investigate whether T2-weighted imaging (T2WI)-based intratumoral and peritumoral radiomics can predict extranodal extension (ENE) and prognosis in patients with resectable rectal cancer.
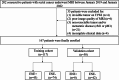
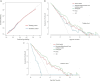
Methods: One hundred sixty-seven patients with resectable rectal cancer including T3T4N + cases were prospectively included. Radiomics features were extracted from intratumoral, peritumoral 3 mm, and peritumoral-mesorectal fat on T2WI images. Least absolute shrinkage and selection operator regression were used for feature selection. A radiomics signature score (Radscore) was built with logistic regression analysis. The area under the receiver operating characteristic curve (AUC) was used to evaluate the performance of each Radscore. A clinical-radiomics nomogram was constructed by the most predictive radiomics signature and clinical risk factors. A prognostic model was constructed by Cox regression analysis to identify 3-year recurrence-free survival (RFS).
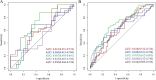
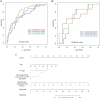
Results: Age, cT stage, and lymph node-irregular border and/or adjacent fat invasion were identified as independent clinical risk factors to construct a clinical model. The nomogram incorporating intratumoral and peritumoral 3 mm Radscore and independent clinical risk factors achieved a better AUC than the clinical model in the training (0.799 vs. 0.736) and validation cohorts (0.723 vs. 0.667). Nomogram-based ENE (hazard ratio [HR] = 2.625, 95% CI = 1.233-5.586, p = 0.012) and extramural vascular invasion (EMVI) (HR = 2.523, 95% CI = 1.247-5.106, p = 0.010) were independent risk factors for predicting 3-year RFS. The prognostic model constructed by these two indicators showed good performance for predicting 3-year RFS in the training (AUC = 0.761) and validation cohorts (AUC = 0.710).
Conclusion: The nomogram incorporating intratumoral and peritumoral 3 mm Radscore and clinical risk factors could predict preoperative ENE. Combining nomogram-based ENE and MRI-reported EMVI may be useful in predicting 3-year RFS.
Critical relevance statement: A clinical-radiomics nomogram could help preoperative predict ENE, and a prognostic model constructed by the nomogram-based ENE and MRI-reported EMVI could predict 3-year RFS in patients with resectable rectal cancer.
Key points: • Intratumoral and peritumoral 3 mm Radscore showed the most capability for predicting ENE. • Clinical-radiomics nomogram achieved the best predictive performance for predicting ENE. • Combining clinical-radiomics based-ENE and EMVI showed good performance for 3-year RFS.
Keywords: Lymph node; Magnetic resonance imaging; Nomograms; Rectal Neoplasms.
© 2024. The Author(s).
Conflict of interest statement
XZ is an employee of GE Healthcare. The remaining authors declare that they have no competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

