Claudin-2 upregulation enhances intestinal permeability, immune activation, dysbiosis, and mortality in sepsis
- PMID: 38412124
- PMCID: PMC10927519
- DOI: 10.1073/pnas.2217877121
Claudin-2 upregulation enhances intestinal permeability, immune activation, dysbiosis, and mortality in sepsis
Abstract
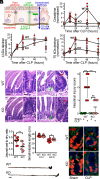
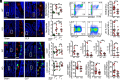
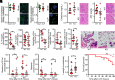
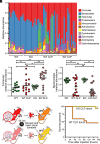
Intestinal epithelial expression of the tight junction protein claudin-2, which forms paracellular cation and water channels, is precisely regulated during development and in disease. Here, we show that small intestinal epithelial claudin-2 expression is selectively upregulated in septic patients. Similar changes occurred in septic mice, where claudin-2 upregulation coincided with increased flux across the paracellular pore pathway. In order to define the significance of these changes, sepsis was induced in claudin-2 knockout (KO) and wild-type (WT) mice. Sepsis-induced increases in pore pathway permeability were prevented by claudin-2 KO. Moreover, claudin-2 deletion reduced interleukin-17 production and T cell activation and limited intestinal damage. These effects were associated with reduced numbers of neutrophils, macrophages, dendritic cells, and bacteria within the peritoneal fluid of septic claudin-2 KO mice. Most strikingly, claudin-2 deletion dramatically enhanced survival in sepsis. Finally, the microbial changes induced by sepsis were less pathogenic in claudin-2 KO mice as survival of healthy WT mice injected with cecal slurry collected from WT mice 24 h after sepsis was far worse than that of healthy WT mice injected with cecal slurry collected from claudin-2 KO mice 24 h after sepsis. Claudin-2 upregulation and increased pore pathway permeability are, therefore, key intermediates that contribute to development of dysbiosis, intestinal damage, inflammation, ineffective pathogen control, and increased mortality in sepsis. The striking impact of claudin-2 deletion on progression of the lethal cascade activated during sepsis suggests that claudin-2 may be an attractive therapeutic target in septic patients.
Keywords: barrier; gut; intestine; sepsis; tight junction.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Evans L., et al. , Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 49, e1063–e1143 (2021). - PubMed
-
- Santacruz C. A., Pereira A. J., Celis E., Vincent J. L., Which multicenter randomized controlled trials in critical care medicine have shown reduced mortality? A systematic review. Crit. Care Med. 47, 1680–1691 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

