Single-cell profiling of African swine fever virus disease in the pig spleen reveals viral and host dynamics
- PMID: 38412127
- PMCID: PMC10927503
- DOI: 10.1073/pnas.2312150121
Single-cell profiling of African swine fever virus disease in the pig spleen reveals viral and host dynamics
Abstract
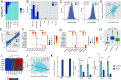
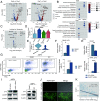
African swine fever, one of the major viral diseases of swine, poses an imminent threat to the global pig industry. The high-efficient replication of the causative agent African swine fever virus (ASFV) in various organs in pigs greatly contributes to the disease. However, how ASFV manipulates the cell population to drive high-efficient replication of the virus in vivo remains unclear. Here, we found that the spleen reveals the most severe pathological manifestation with the highest viral loads among various organs in pigs during ASFV infection. By using single-cell-RNA-sequencing technology and multiple methods, we determined that macrophages and monocytes are the major cell types infected by ASFV in the spleen, showing high viral-load heterogeneity. A rare subpopulation of immature monocytes represents the major population infected at late infection stage. ASFV causes massive death of macrophages, but shifts its infection into these monocytes which significantly arise after the infection. The apoptosis, interferon response, and antigen-presentation capacity are inhibited in these monocytes which benefits prolonged infection of ASFV in vivo. Until now, the role of immature monocytes as an important target by ASFV has been overlooked due to that they do not express classical monocyte marker CD14. The present study indicates that the shift of viral infection from macrophages to the immature monocytes is critical for maintaining prolonged ASFV infection in vivo. This study sheds light on ASFV tropism, replication, and infection dynamics, and elicited immune response, which may instruct future research on antiviral strategies.
Keywords: African swine fever virus; cellular tropism; host antiviral response; monocytes; single-cell RNA sequencing.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Gogin A., Gerasimov V., Malogolovkin A., Kolbasov D., African swine fever in the North Caucasus region and the Russian Federation in years 2007–2012. Virus Res. 173, 198–203 (2013). - PubMed
-
- Kim H. J., et al. , Outbreak of African swine fever in South Korea, 2019. Transbound Emerg. Dis. 67, 473–475 (2020). - PubMed
-
- Blome S., Gabriel C., Beer M., Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res. 173, 122–130 (2013). - PubMed
-
- Dixon L., Sun H., Roberts H., African swine fever. Antiviral Res. 165, 34–41 (2019). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

