Immunoinformatics-guided recombinant polypeptide-based enzyme-linked immunosorbent assay for seromonitoring of laboratory animals for minute virus of mice and Kilham rat virus
- PMID: 38412152
- PMCID: PMC10898725
- DOI: 10.1371/journal.pone.0298742
Immunoinformatics-guided recombinant polypeptide-based enzyme-linked immunosorbent assay for seromonitoring of laboratory animals for minute virus of mice and Kilham rat virus
Abstract
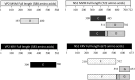
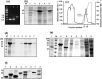
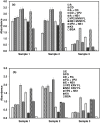
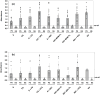
Subclinical infection of laboratory animals with one or more of several pathogens affects the results of experiments on animals. Monitoring the health of laboratory animals encompasses routine surveillance for pathogens, including several viruses. This study aimed to explore the development of an alternative assay to the existing ones for detecting infection of mice and rats with the parvoviruses minute virus of mice (MVM) and Kilham rat virus (KRV), respectively. Full-length VP2 and NS1 proteins of these parvoviruses, besides fragments containing multiple predicted epitopes stitched together, were studied for serological detection. The optimal dilution of full-length proteins and antigenic regions containing predicted epitopes for coating, test sera, and conjugate was determined using a checkerboard titration at each step. The assays were evaluated vis-à-vis commercially available ELISA kits. The results showed that an engineered fusion of fragments containing multiple predicted MVM VP2 and NS1 epitopes was better than either of the full-length proteins for detecting antibodies in 90% of the tested sera samples. For KRV ELISA, full-length VP2 was better compared to other individual recombinant protein fragments or combinations thereof for the detection of antibodies in sera. This report is the first description of an ELISA for KRV and an improved assay for MVM. Importantly, our assays could be exploited with small volumes of sera. The results also demonstrate the utility of immunoinformatics-driven polypeptide engineering in the development of diagnostic assays and the potential to develop better tests for monitoring the health status of laboratory animals.
Copyright: © 2024 Kaur et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Validation of an enzyme-linked immunosorbent assay for detection of mouse parvovirus infection in laboratory mice.Comp Med. 2002 Apr;52(2):160-6. Comp Med. 2002. PMID: 12022396
-
Development of ELISA using recombinant antigens for specific detection of mouse parvovirus infection.Exp Anim. 2006 Apr;55(2):117-24. doi: 10.1538/expanim.55.117. Exp Anim. 2006. PMID: 16651694
-
Serodiagnosis of mice minute virus and mouse parvovirus infections in mice by enzyme-linked immunosorbent assay with baculovirus-expressed recombinant VP2 proteins.Clin Diagn Lab Immunol. 2002 Sep;9(5):1025-31. doi: 10.1128/cdli.9.5.1025-1031.2002. Clin Diagn Lab Immunol. 2002. PMID: 12204954 Free PMC article.
-
Enzyme-linked immunosorbent assay in the diagnosis of Kilham rat virus infection in rats.Lab Anim. 1984 Oct;18(4):364-70. doi: 10.1258/002367784780865289. Lab Anim. 1984. PMID: 6096629
-
Epidemiological and phylogenetic analysis of institutional mouse parvoviruses.Exp Mol Pathol. 2013 Aug;95(1):32-7. doi: 10.1016/j.yexmp.2013.03.009. Epub 2013 Mar 29. Exp Mol Pathol. 2013. PMID: 23545399
References
-
- Rowe WP, Hartley JW, Huebner RJ. Polyoma and Other Indigenous Mouse Viruses. Lab Anim Care. 1963;13:SUPPL166–75. . - PubMed
-
- Descoteaux JP, Grignon-Archambault D, Lussier G. Serologic study on the prevalence of murine viruses in five Canadian mouse colonies. Lab Anim Sci. 1977;27(5 Pt 1):621–6. . - PubMed
-
- Casebolt DB, Lindsey JR, Cassell GH. Prevalence rates of infectious agents among commercial breeding populations of rats and mice. Lab Anim Sci. 1988;38(3):327–9. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

