Standardizing to specific target populations in distributed networks and multisite pharmacoepidemiologic studies
- PMID: 38412261
- PMCID: PMC11520739
- DOI: 10.1093/aje/kwae015
Standardizing to specific target populations in distributed networks and multisite pharmacoepidemiologic studies
Abstract
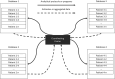
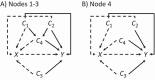
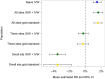
Distributed network studies and multisite studies assess drug safety and effectiveness in diverse populations by pooling information. Targeting groups of clinical or policy interest (including specific sites or site combinations) and applying weights based on effect measure modifiers (EMMs) prior to pooling estimates within multisite studies may increase interpretability and improve precision. We simulated a 4-site study, standardized each site using inverse odds weights (IOWs) to resemble the 3 smallest sites or the smallest site, estimated IOW-weighted risk differences (RDs), and combined estimates with inverse variance weights (IVWs). We also created an artificial distributed network in the Clinical Practice Research Datalink (CPRD) Aurum consisting of 1 site for each geographic region. We compared metformin and sulfonylurea initiators with respect to mortality, targeting the smallest region. In the simulation, IOWs reduced differences between estimates and increased precision when targeting the 3 smallest sites or the smallest site. In the CPRD Aurum study, the IOW + IVW estimate was also more precise (smallest region: RD = 5.41% [95% CI, 1.03-9.79]; IOW + IVW estimate: RD = 3.25% [95% CI, 3.07-3.43]). When performing pharmacoepidemiologic research in distributed networks or multisite studies in the presence of EMMs, designation of target populations has the potential to improve estimate precision and interpretability. This article is part of a Special Collection on Pharmacoepidemiology.
Keywords: distributed networks; external validity; standardization; target populations.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

