Timing of Coronavirus Disease 2019 (COVID-19) Vaccination and Effects on Menstrual Cycle Changes
- PMID: 38412506
- PMCID: PMC10953681
- DOI: 10.1097/AOG.0000000000005550
Timing of Coronavirus Disease 2019 (COVID-19) Vaccination and Effects on Menstrual Cycle Changes
Abstract
Objective: To assess whether menstrual cycle timing (follicular or luteal phase) of coronavirus disease 2019 (COVID-19) vaccine administration is associated with cycle length changes.
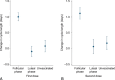
Methods: We used prospectively collected (2021-2022) menstrual cycle tracking data from 19,497 reproductive-aged users of the application "Natural Cycles." We identified whether vaccine was delivered in the follicular or luteal phase and also included an unvaccinated control group. Our primary outcome was the adjusted within-individual change in cycle length (in days) from the average of the three menstrual cycles before the first vaccination cycle (individuals in the unvaccinated control group were assigned a notional vaccine date). We also assessed cycle length changes in the second vaccination cycle and whether a clinically significant change in cycle length (8 days or more) occurred in either cycle.
Results: Most individuals were younger than age 35 years (80.1%) and from North America (28.6%), continental Europe (33.5%), or the United Kingdom (31.7%). In the vaccinated group, the majority received an mRNA vaccine (63.8% of the full sample). Individuals vaccinated in the follicular phase experienced an average 1-day longer adjusted cycle length with a first or second dose of COVID-19 vaccine compared with their prevaccination average (first dose: 1.00 day [98.75% CI, 0.88-1.13], second dose: 1.11 days [98.75% CI, 0.93-1.29]); those vaccinated in the luteal phase and those in the unvaccinated control group experienced no change in cycle length (respectively, first dose: -0.09 days [98.75% CI, -0.26 to 0.07], second dose: 0.06 days [98.75% CI, -0.16 to 0.29], unvaccinated notional first dose: 0.08 days [98.75% CI, -0.10 to 0.27], second dose: 0.17 days [98.75% CI, -0.04 to 0.38]). Those vaccinated during the follicular phase were also more likely to experience a clinically significant change in cycle length (8 days or more; first dose: 6.8%) than those vaccinated in the luteal phase or unvaccinated (3.3% and 5.0%, respectively; P <.001).
Conclusion: COVID-19 vaccine-related cycle length increases are associated with receipt of vaccination in the first half of the menstrual cycle (follicular phase).
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Financial Disclosure Alison Edelman reports honoraria and/or travel reimbursement from ACOG, WHO, CDC and Gynuity for committee activities. Alison Edelman receives royalties from UpToDate, Inc. Oregon Health & Science University (OHSU) receives research funding from OHSU Foundation, Merck, HRA Pharma, and NIH for which Alison Edelman is the principal investigator. Blair G. Darney reports honoraria and travel reimbursement from ACOG and SFP for board, committee, and mentorship activities. OHSU receives research funding from Merck/Organon and OPA/DHHS for which Blair G. Darney is the principal investigator. OHSU receives research funding from OHSU foundation, the Bill & Melinda Gates Foundation, ABOG, ASRM and the NIH for which Leo Han is the principal investigator. Eleonora Benhar, Agathe Van Lamsweerde, and Jack T. Pearson are employees of Natural Cycles. Natural Cycles received cost reimbursement from grant funds for data processing and secure transfer. Kristen A. Matteson reports honoraria and travel reimbursement from ABOG and travel reimbursement from ACOG. Women and Infants Hospital received funding from Myovant for consulting work done by Kristen A. Matteson on outcomes measures for heavy menstrual bleeding. Victoria Male reports research funding from Borne, payment for acting as an external examiner for the Universities of Cambridge, Leeds and Swansea, and Trinity College Dublin, royalties received for contribution to Immunology 9th edition (Elsevier), payment for articles in the Guardian newspaper and travel reimbursement for attending the 16th Vaccine Congress (Elsevier). Sharon Cameron receives research funding for a contraceptive focused study from FHI360. Emily R. Boniface did not report any potential conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

