PXPermute reveals staining importance in multichannel imaging flow cytometry
- PMID: 38412831
- PMCID: PMC10921034
- DOI: 10.1016/j.crmeth.2024.100715
PXPermute reveals staining importance in multichannel imaging flow cytometry
Abstract
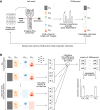
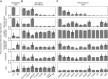
Imaging flow cytometry (IFC) allows rapid acquisition of numerous single-cell images per second, capturing information from multiple fluorescent channels. However, the traditional process of staining cells with fluorescently labeled conjugated antibodies for IFC analysis is time consuming, expensive, and potentially harmful to cell viability. To streamline experimental workflows and reduce costs, it is crucial to identify the most relevant channels for downstream analysis. In this study, we introduce PXPermute, a user-friendly and powerful method for assessing the significance of IFC channels, particularly for cell profiling. Our approach evaluates channel importance by permuting pixel values within each channel and analyzing the resulting impact on machine learning or deep learning models. Through rigorous evaluation of three multichannel IFC image datasets, we demonstrate PXPermute's potential in accurately identifying the most informative channels, aligning with established biological knowledge. PXPermute can assist biologists with systematic channel analysis, experimental design optimization, and biomarker identification.
Keywords: CP: Imaging; CP: Systems biology; cell profiling; channel importance; computer vision; deep learning; image flow cytometry; interpretable artificial intelligence; machine learning; staining importance.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

