High Flow Nasal Cannula for Weaning Nasal Continuous Positive Airway Pressure in Preterm Infants: A Systematic Review and Meta-Analysis
- PMID: 38412846
- PMCID: PMC11151992
- DOI: 10.1159/000536464
High Flow Nasal Cannula for Weaning Nasal Continuous Positive Airway Pressure in Preterm Infants: A Systematic Review and Meta-Analysis
Abstract
Introduction: The aim of this study was to systematically review the benefits and harms of using a high-flow nasal cannula (HFNC) for weaning continuous positive airway pressure (CPAP) support in preterm infants.
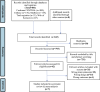
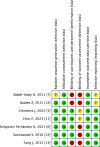
Methods: Cochrane Central, EMBASE, Medline, and Web of Science were searched from inception to July 15, 2023. Randomised clinical trials (RCTs) comparing weaning CPAP using HFNC versus weaning CPAP alone and evaluating predefined outcomes were included. Two authors independently performed data extraction and methodological quality assessment. Meta-analysis was conducted using a random-effects model, and the certainty of evidence was assessed using Cochrane GRADE.
Results: Among 843 identified records, seven RCTs involving 781 preterm infants were eligible for analysis. The meta-analysis found no statistically significant difference in duration of respiratory support when using HFNC for weaning compared to weaning CPAP alone (mean difference (95% confidence interval) 3.52 (-0.02, 7.05); 5 RCTs; participants = 488; I2 = 29%). The evidence certainty was downgraded to low due to study limitations and imprecision. There were no significant differences in secondary outcomes, except for a lower occurrence of nasal trauma with HFNC for weaning CPAP compared to weaning CPAP alone (relative risk (95% confidence interval) 0.61 (0.38, 0.99); 4 RCTs; participants = 335; I2 = 0%). The evidence certainty for the secondary outcomes was low to very low.
Conclusion: Low certainty of evidence suggests using HFNC for weaning CPAP in preterm infants may not impact the duration of respiratory support. Caution is advised when considering HFNC for weaning CPAP, especially in extremely preterm infants, until additional supportive evidence on its safety becomes available.
Keywords: Continuous positive airway pressure; Heated humidified high flow nasal cannula; Neonatal intensive care unit; Preterm infants; Weaning.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
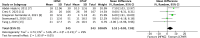
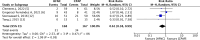
References
-
- Jardine LA, Inglis GD, Davies MW. Strategies for the withdrawal of nasal continuous positive airway pressure (nCPAP) in preterm infants. Cochrane Database Syst Rev. 2011(2):CD006979. - PubMed
-
- Osman M, Elsharkawy A, Abdel-Hady H. Assessment of pain during application of nasal continuous positive airway pressure and heated, humidified high-flow nasal cannulae in preterm infants. J Perinatol. 2014 2015;35(4):263–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

