Impaired cerebellar plasticity hypersensitizes sensory reflexes in SCN2A-associated ASD
- PMID: 38412857
- PMCID: PMC11065582
- DOI: 10.1016/j.neuron.2024.01.029
Impaired cerebellar plasticity hypersensitizes sensory reflexes in SCN2A-associated ASD
Abstract
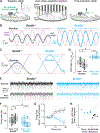
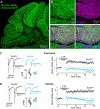
Children diagnosed with autism spectrum disorder (ASD) commonly present with sensory hypersensitivity or abnormally strong reactions to sensory stimuli. Such hypersensitivity can be overwhelming, causing high levels of distress that contribute markedly to the negative aspects of the disorder. Here, we identify a mechanism that underlies hypersensitivity in a sensorimotor reflex found to be altered in humans and in mice with loss of function in the ASD risk-factor gene SCN2A. The cerebellum-dependent vestibulo-ocular reflex (VOR), which helps maintain one's gaze during movement, was hypersensitized due to deficits in cerebellar synaptic plasticity. Heterozygous loss of SCN2A-encoded NaV1.2 sodium channels in granule cells impaired high-frequency transmission to Purkinje cells and long-term potentiation, a form of synaptic plasticity important for modulating VOR gain. VOR plasticity could be rescued in mice via a CRISPR-activator approach that increases Scn2a expression, demonstrating that evaluation of a simple reflex can be used to assess and quantify successful therapeutic intervention.
Keywords: autism spectrum disorder; cerebellum; gene therapy; human; mouse; neurodevelopmental disorder; sodium channel; vestibulo-ocular reflex.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests N.A. is the cofounder and on the scientific advisory board of Regel Tx. K.J.B. is on the scientific advisory board of Regel Tx. N.A. and K.J.B. receive funding from BioMarin Pharmaceutical Incorporated.
Figures




Update of
-
Impaired cerebellar plasticity hypersensitizes sensory reflexes in SCN2A-associated ASD.bioRxiv [Preprint]. 2023 Jun 7:2023.06.05.543814. doi: 10.1101/2023.06.05.543814. bioRxiv. 2023. Update in: Neuron. 2024 May 1;112(9):1444-1455.e5. doi: 10.1016/j.neuron.2024.01.029. PMID: 37333267 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

