Stabilizing Azaheptacenes
- PMID: 38413006
- PMCID: PMC10921409
- DOI: 10.1021/jacs.3c13629
Stabilizing Azaheptacenes
Abstract
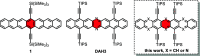
The symmetrical 7,16-diaza-6,8,15,17-tetrakis(triisopropylsilylethynyl)heptacene was obtained by a Pd-catalyzed reaction of a 2,3-diamino-1,4-diethynylanthracene with a 2,3-dibromo-1,4-diethynyl anthracene. Positioning the TIPS-ethynyl groups adjacent to the central ring suppresses dimerization via [4+4] cycloadditions and Diels-Alder reactions; the middle pyrazine ring renders this species stable to oxidation. A single crystal structure was obtained, and thin film transistors with μn = 0.042 cm2 V-1 s-1 were produced. Transposition of the alkynyl groups into the 5,8,15,18-positions with a quinoxaline unit in the center of the heptacene decreases the stability, as does the introduction of two more nitrogen atoms into the 5,18-positions. The hydrocarbon 6,8,15,17-tetrakis(triisopropylsilylethynyl)heptacene is reasonably stable with a half-life of 25 h in solution. Four correctly placed TIPS-ethynyl groups protect heptacene cores.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Kitamura M.; Arakawa Y. Pentacene-based organic field-effect transistors. J. Phys.: Condens. Matter 2008, 20, 18401110.1088/0953-8984/20/18/184011. - DOI
-
- Ruiz R.; Choudhary D.; Nickel B.; Toccoli T.; Chang K.-C.; Mayer A. C.; Clancy P.; Blakely J. M.; Headrick R. L.; Iannotta S. Pentacene thin film growth. Chem. Mater. 2004, 16, 4497–4508. 10.1021/cm049563q. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

