Mycophenolate and azathioprine efficacy in interstitial lung disease: a systematic review and meta-analysis
- PMID: 38413120
- PMCID: PMC10973691
- DOI: 10.1136/bmjresp-2023-002163
Mycophenolate and azathioprine efficacy in interstitial lung disease: a systematic review and meta-analysis
Abstract
Objectives: Mycophenolate mofetil (MMF) and azathioprine (AZA) are immunomodulatory treatments in interstitial lung disease (ILD). This systematic review aimed to evaluate the efficacy of MMF or AZA on pulmonary function in ILD.
Design: Population included any ILD diagnosis, intervention included MMF or AZA treatment, outcome was delta change from baseline in per cent predicted forced vital capacity (%FVC) and gas transfer (diffusion lung capacity of carbon monoxide, %DLco). The primary endpoint compared outcomes relative to placebo comparator, the secondary endpoint assessed outcomes in treated groups only.
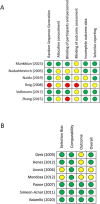
Eligibility criteria: Randomised controlled trials (RCTs) and prospective observational studies were included. No language restrictions were applied. Retrospective studies and studies with high-dose concomitant steroids were excluded.
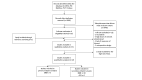
Data synthesis: The systematic search was performed on 9 May. Meta-analyses according to drug and outcome were specified with random effects, I2 evaluated heterogeneity and Grading of Recommendations, Assessment, Development and Evaluation evaluated certainty of evidence. Primary endpoint analysis was restricted to RCT design, secondary endpoint included subgroup analysis according to prospective observational or RCT design.
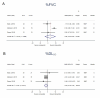
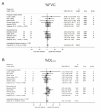
Results: A total of 2831 publications were screened, 12 were suitable for quantitative synthesis. Three MMF RCTs were included with no significant effect on the primary endpoints (%FVC 2.94, 95% CI -4.00 to 9.88, I2=79.3%; %DLco -2.03, 95% CI -4.38 to 0.32, I2=0.0%). An overall 2.03% change from baseline in %FVC (95% CI 0.65 to 3.42, I2=0.0%) was observed in MMF, and RCT subgroup summary estimated a 4.42% change from baseline in %DLCO (95% CI 2.05 to 6.79, I2=0.0%). AZA studies were limited. All estimates were considered very low certainty evidence.
Conclusions: There were limited RCTs of MMF or AZA and their benefit in ILD was of very low certainty. MMF may support preservation of pulmonary function, yet confidence in the effect was weak. To support high certainty evidence, RCTs should be designed to directly assess MMF efficacy in ILD.
Prospero registration number: CRD42023423223.
Keywords: Interstitial Fibrosis; Respiratory Function Test.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GJ is supported by a National Institute for Health Research (NIHR) Research Professorship (NIHR reference RP-2017-08-ST2-014). GJ is a trustee of Action for Pulmonary Fibrosis and reports personal fees from Astra Zeneca, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Chiesi, Daewoong, Galapagos, Galecto, GlaxoSmithKline, Heptares, NuMedii, PatientMPower, Pliant, Promedior, Redx, Resolution Therapeutics, Roche, Veracyte and Vicore. CJR reports grants from Boehringer Ingelheim, and honoraria or consulting fees from Boehringer Ingelheim, Pliant Therapeutics, Astra Zeneca, Trevi Therapeutics, Veracyte, Hoffmann-La Roche, Cipla. FL, IS, LF, WA and LK-D report no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
