Targeting the activated microenvironment with endosialin (CD248)-directed CAR-T cells ablates perivascular cells to impair tumor growth and metastasis
- PMID: 38413223
- PMCID: PMC10900351
- DOI: 10.1136/jitc-2023-008608
Targeting the activated microenvironment with endosialin (CD248)-directed CAR-T cells ablates perivascular cells to impair tumor growth and metastasis
Abstract
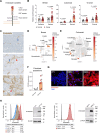
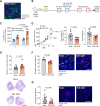
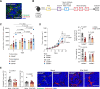
Background: Targeting of solid cancers with chimeric antigen receptor (CAR)-T cells is limited by the lack of suitable tumor-specific antigens and the immunosuppressive, desmoplastic tumor microenvironment that impedes CAR-T cell infiltration, activity and persistence. We hypothesized that targeting the endosialin (CD248) receptor, strongly expressed by tumor-associated pericytes and perivascular cancer-associated fibroblasts, would circumvent these challenges and offer an exciting antigen for CAR-T cell therapy due to the close proximity of target cells to the tumor vasculature, the limited endosialin expression in normal tissues and the lack of phenotype observed in endosialin knockout mice.
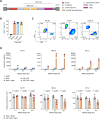
Methods: We generated endosialin-directed E3K CAR-T cells from three immunocompetent mouse strains, BALB/c, FVB/N and C57BL/6. E3K CAR-T cell composition (CD4+/CD8+ ratio), activity in vitro against endosialin+ and endosialin- cells, and expansion and activity in vivo in syngeneic tumor models as well as in tumor-naive healthy and wounded mice and tumor-bearing endosialin knockout mice was assessed.
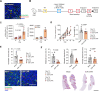
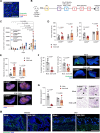
Results: E3K CAR-T cells were active in vitro against both mouse and human endosialin+, but not endosialin-, cells. Adoptively transferred E3K CAR-T cells exhibited no activity in endosialin knockout mice, tumor-naive endosialin wildtype mice or in wound healing models, demonstrating an absence of off-target and on-target/off-tumor activity. By contrast, adoptive transfer of E3K CAR-T cells into BALB/c, FVB/N or C57BL/6 mice bearing syngeneic breast or lung cancer lines depleted target cells in the tumor stroma resulting in increased tumor necrosis, reduced tumor growth and a substantial impairment in metastatic outgrowth.
Conclusions: Together these data highlight endosialin as a viable antigen for CAR-T cell therapy and that targeting stromal cells closely associated with the tumor vasculature avoids CAR-T cells having to navigate the harsh immunosuppressive tumor microenvironment. Further, the ability of E3K CAR-T cells to recognize and target both mouse and human endosialin+ cells makes a humanized and optimized E3K CAR a promising candidate for clinical development applicable to a broad range of solid tumor types.
Keywords: Breast Cancer; Chimeric antigen receptor - CAR; Lung Cancer; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Endosialin-Expressing Pericytes Promote Metastatic Dissemination.Cancer Res. 2016 Sep 15;76(18):5313-25. doi: 10.1158/0008-5472.CAN-16-0932. Cancer Res. 2016. PMID: 27635044
-
Endosialin in Cancer: Expression Patterns, Mechanistic Insights, and Therapeutic Approaches.Theranostics. 2024 Jan 1;14(1):379-391. doi: 10.7150/thno.89495. eCollection 2024. Theranostics. 2024. PMID: 38164138 Free PMC article. Review.
-
Endosialin/TEM 1/CD248 is a pericyte marker of embryonic and tumor neovascularization.Microvasc Res. 2008 Nov;76(3):180-8. doi: 10.1016/j.mvr.2008.07.008. Epub 2008 Aug 8. Microvasc Res. 2008. PMID: 18761022
-
Endosialin-directed CAR-T cell therapy: A promising approach for targeting triple-negative breast cancer.Biochim Biophys Acta Mol Basis Dis. 2025 Aug;1871(6):167852. doi: 10.1016/j.bbadis.2025.167852. Epub 2025 May 1. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 40318845 Review.
-
Endosialin-positive CAFs promote hepatocellular carcinoma progression by suppressing CD8+ T cell infiltration.J Immunother Cancer. 2024 Sep 10;12(9):e009111. doi: 10.1136/jitc-2024-009111. J Immunother Cancer. 2024. PMID: 39260826 Free PMC article.
Cited by
-
Tumour-associated vasculature in T cell homing and immunity: opportunities for cancer therapy.Nat Rev Immunol. 2025 Jun 27. doi: 10.1038/s41577-025-01187-w. Online ahead of print. Nat Rev Immunol. 2025. PMID: 40579467 Review.
-
SCAN-ACT: adoptive T cell therapy target discovery through single-cell transcriptomics.Genome Med. 2025 Aug 14;17(1):89. doi: 10.1186/s13073-025-01514-9. Genome Med. 2025. PMID: 40814001 Free PMC article.
-
Pan-cancer analysis identified CD248 as a potential target for multiple tumor types.Front Pharmacol. 2025 Apr 10;16:1554632. doi: 10.3389/fphar.2025.1554632. eCollection 2025. Front Pharmacol. 2025. PMID: 40276611 Free PMC article.
-
Microvascularization of the Vocal Folds: Molecular Architecture, Functional Insights, and Personalized Research Perspectives.J Pers Med. 2025 Jul 7;15(7):293. doi: 10.3390/jpm15070293. J Pers Med. 2025. PMID: 40710410 Free PMC article. Review.
-
The potential role of CD8+ cytotoxic T lymphocytes and one branch connected with tissue-resident memory in non-luminal breast cancer.PeerJ. 2024 Jul 10;12:e17667. doi: 10.7717/peerj.17667. eCollection 2024. PeerJ. 2024. PMID: 39006029 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
