Cell cycle heterogeneity and plasticity of colorectal cancer stem cells
- PMID: 38413370
- PMCID: PMC11093209
- DOI: 10.1111/cas.16117
Cell cycle heterogeneity and plasticity of colorectal cancer stem cells
Abstract
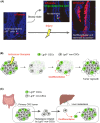
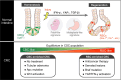
Cancer stem cells (CSCs) are a long-lived and self-renewing cancer cell population that drives tumor propagation and maintains cancer heterogeneity. They are also implicated in the therapeutic resistance of various types of cancer. Recent studies of CSCs in colorectal cancer (CRC) have uncovered fundamental paradigms that have increased understanding of CSC systems in solid tumors. Colorectal CSCs share multiple biological properties with normal intestinal stem cells (ISCs), including expression of the stem cell marker Lgr5. New evidence suggests that colorectal CSCs manifest substantial heterogeneity, as exemplified by the existence of both actively cycling Lgr5+ CSCs as well as quiescent Lgr5+ CSCs that are resistant to conventional anticancer therapies. The classical view of a rigid cell hierarchy and irreversible cell differentiation trajectory in normal and neoplastic tissues is now challenged by the finding that differentiated cells have the capacity to revert to stem cells through dynamic physiological reprogramming events. Such plasticity of CSC systems likely underlies both carcinogenesis and therapeutic resistance in CRC. Further characterization of the mechanisms underpinning the heterogeneity and plasticity of CSCs should inform future development of eradicative therapeutic strategies for CRC.
Keywords: cancer stem cell; colorectal cancer; intestinal stem cell; plasticity; quiescence.
© 2024 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Keiichi I. Nakayama is an editorial board member of Cancer Science. The other author has no conflict of interest.
Figures


Similar articles
-
Colorectal Cancer Stem Cells Acquire Chemoresistance Through the Upregulation of F-Box/WD Repeat-Containing Protein 7 and the Consequent Degradation of c-Myc.Stem Cells. 2017 Sep;35(9):2027-2036. doi: 10.1002/stem.2668. Epub 2017 Jul 31. Stem Cells. 2017. PMID: 28699179
-
Co-expression of Lgr5 and CXCR4 characterizes cancer stem-like cells of colorectal cancer.Oncotarget. 2016 Dec 6;7(49):81144-81155. doi: 10.18632/oncotarget.13214. Oncotarget. 2016. PMID: 27835894 Free PMC article.
-
Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells.Stem Cells. 2012 Nov;30(11):2378-86. doi: 10.1002/stem.1233. Stem Cells. 2012. PMID: 22969042
-
Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting.BMB Rep. 2017 Mar;50(3):117-125. doi: 10.5483/bmbrep.2017.50.3.222. BMB Rep. 2017. PMID: 27998397 Free PMC article. Review.
-
The Roles of Cancer Stem Cells and Therapy Resistance in Colorectal Carcinoma.Cells. 2020 Jun 3;9(6):1392. doi: 10.3390/cells9061392. Cells. 2020. PMID: 32503256 Free PMC article. Review.
Cited by
-
Lipid metabolism dynamics in cancer stem cells: potential targets for cancers.Front Pharmacol. 2024 Jun 27;15:1367981. doi: 10.3389/fphar.2024.1367981. eCollection 2024. Front Pharmacol. 2024. PMID: 38994204 Free PMC article. Review.
-
Drug repositioning for pan-cancers of the digestive system: Identification of amonafide and BX795 as potential therapeutics via integrative Omics analysis.PLoS One. 2025 Jun 16;20(6):e0325700. doi: 10.1371/journal.pone.0325700. eCollection 2025. PLoS One. 2025. PMID: 40522954 Free PMC article.
-
Mechanisms Behind the Impact of PIWI Proteins on Cancer Cells: Literature Review.Int J Mol Sci. 2024 Nov 14;25(22):12217. doi: 10.3390/ijms252212217. Int J Mol Sci. 2024. PMID: 39596284 Free PMC article. Review.
-
Molecular and phenotypic characterization of 5-FU resistant colorectal cancer cells: toward enrichment of cancer stem cells.Cancer Cell Int. 2025 Apr 18;25(1):154. doi: 10.1186/s12935-025-03758-2. Cancer Cell Int. 2025. PMID: 40251609 Free PMC article.
-
Novel Lysosomal-Associated Transmembrane Protein 4B-Positive Stem-Like Cell Subpopulation Characterizes High-Risk Colorectal Cancer Subtypes.MedComm (2020). 2025 Jul 13;6(7):e70284. doi: 10.1002/mco2.70284. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40661135 Free PMC article.
References
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105‐111. - PubMed
-
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645‐648. - PubMed
-
- Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124‐1134. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

