Bicarbonate signalling via G protein-coupled receptor regulates ischaemia-reperfusion injury
- PMID: 38413581
- PMCID: PMC10899177
- DOI: 10.1038/s41467-024-45579-3
Bicarbonate signalling via G protein-coupled receptor regulates ischaemia-reperfusion injury
Abstract
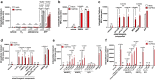
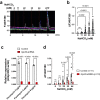
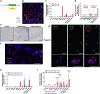
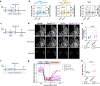
Homoeostatic regulation of the acid-base balance is essential for cellular functional integrity. However, little is known about the molecular mechanism through which the acid-base balance regulates cellular responses. Here, we report that bicarbonate ions activate a G protein-coupled receptor (GPCR), i.e., GPR30, which leads to Gq-coupled calcium responses. Gpr30-Venus knock-in mice reveal predominant expression of GPR30 in brain mural cells. Primary culture and fresh isolation of brain mural cells demonstrate bicarbonate-induced, GPR30-dependent calcium responses. GPR30-deficient male mice are protected against ischemia-reperfusion injury by a rapid blood flow recovery. Collectively, we identify a bicarbonate-sensing GPCR in brain mural cells that regulates blood flow and ischemia-reperfusion injury. Our results provide a perspective on the modulation of GPR30 signalling in the development of innovative therapies for ischaemic stroke. Moreover, our findings provide perspectives on acid/base sensing GPCRs, concomitantly modulating cellular responses depending on fluctuating ion concentrations under the acid-base homoeostasis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Brinkman, J. E. & Sharma, S. Physiology, metabolic alkalosis. (StatPearls Publishing, 2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

