Hyperfunction of post-synaptic density protein 95 promotes seizure response in early-stage aβ pathology
- PMID: 38413732
- PMCID: PMC10933348
- DOI: 10.1038/s44319-024-00090-0
Hyperfunction of post-synaptic density protein 95 promotes seizure response in early-stage aβ pathology
Abstract
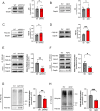
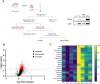
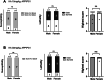
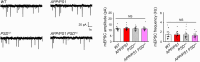
Accumulation of amyloid-beta (Aβ) can lead to the formation of aggregates that contribute to neurodegeneration in Alzheimer's disease (AD). Despite globally reduced neural activity during AD onset, recent studies have suggested that Aβ induces hyperexcitability and seizure-like activity during the early stages of the disease that ultimately exacerbate cognitive decline. However, the underlying mechanism is unknown. Here, we reveal an Aβ-induced elevation of postsynaptic density protein 95 (PSD-95) in cultured neurons in vitro and in an in vivo AD model using APP/PS1 mice at 8 weeks of age. Elevation of PSD-95 occurs as a result of reduced ubiquitination caused by Akt-dependent phosphorylation of E3 ubiquitin ligase murine-double-minute 2 (Mdm2). The elevation of PSD-95 is consistent with the facilitation of excitatory synapses and the surface expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors induced by Aβ. Inhibition of PSD-95 corrects these Aβ-induced synaptic defects and reduces seizure activity in APP/PS1 mice. Our results demonstrate a mechanism underlying elevated seizure activity during early-stage Aβ pathology and suggest that PSD-95 could be an early biomarker and novel therapeutic target for AD.
Keywords: AMPA Receptors; Alzheimer’s Disease; Mdm2; PSD-95; Seizure.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses.Neurobiol Dis. 2005 Nov;20(2):187-98. doi: 10.1016/j.nbd.2005.02.008. Neurobiol Dis. 2005. PMID: 16242627
-
A Class I HDAC Inhibitor Rescues Synaptic Damage and Neuron Loss in APP-Transfected Cells and APP/PS1 Mice through the GRIP1/AMPA Pathway.Molecules. 2022 Jun 29;27(13):4160. doi: 10.3390/molecules27134160. Molecules. 2022. PMID: 35807406 Free PMC article.
-
Non-fibrillar beta-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors.Eur J Neurosci. 2006 Apr;23(8):2035-47. doi: 10.1111/j.1460-9568.2006.04733.x. Eur J Neurosci. 2006. PMID: 16630051
-
The inside-out amyloid hypothesis and synapse pathology in Alzheimer's disease.Neurodegener Dis. 2014;13(2-3):142-6. doi: 10.1159/000354776. Epub 2013 Sep 24. Neurodegener Dis. 2014. PMID: 24080821 Review.
-
APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis.Brain Struct Funct. 2010 Mar;214(2-3):111-26. doi: 10.1007/s00429-009-0232-6. Epub 2009 Nov 29. Brain Struct Funct. 2010. PMID: 20091183 Free PMC article. Review.
Cited by
-
Alzheimer's Disease and Epilepsy: Exploring Shared Pathways and Promising Biomarkers for Future Treatments.J Clin Med. 2024 Jul 1;13(13):3879. doi: 10.3390/jcm13133879. J Clin Med. 2024. PMID: 38999445 Free PMC article. Review.
-
Longitudinal pharmacokinetic and safety studies for dose optimization of a brain- penetrating erythropoietin for Alzheimer's disease.Res Sq [Preprint]. 2025 Jun 17:rs.3.rs-6874797. doi: 10.21203/rs.3.rs-6874797/v1. Res Sq. 2025. PMID: 40585228 Free PMC article. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

