A functional personalised oncology approach against metastatic colorectal cancer in matched patient derived organoids
- PMID: 38413740
- PMCID: PMC10899621
- DOI: 10.1038/s41698-024-00543-8
A functional personalised oncology approach against metastatic colorectal cancer in matched patient derived organoids
Abstract
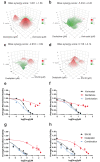
Globally, colorectal cancer (CRC) is the third most frequently occurring cancer. Progression on to an advanced metastatic malignancy (metCRC) is often indicative of poor prognosis, as the 5-year survival rates of patients decline rapidly. Despite the availability of many systemic therapies for the management of metCRC, the long-term efficacies of these regimens are often hindered by the emergence of treatment resistance due to intratumoral and intertumoral heterogeneity. Furthermore, not all systemic therapies have associated biomarkers that can accurately predict patient responses. Hence, a functional personalised oncology (FPO) approach can enable the identification of patient-specific combinatorial vulnerabilities and synergistic combinations as effective treatment strategies. To this end, we established a panel of CRC patient-derived organoids (PDOs) as clinically relevant biological systems, of which three pairs of matched metCRC PDOs were derived from the primary sites (ptCRC) and metastatic lesions (mCRC). Histological and genomic characterisation of these PDOs demonstrated the preservation of histopathological and genetic features found in the parental tumours. Subsequent application of the phenotypic-analytical drug combination interrogation platform, Quadratic Phenotypic Optimisation Platform, in these pairs of PDOs identified patient-specific drug sensitivity profiles to epigenetic-based combination therapies. Most notably, matched PDOs from one patient exhibited differential sensitivity patterns to the rationally designed drug combinations despite being genetically similar. These findings collectively highlight the limitations of current genomic-driven precision medicine in guiding treatment strategies for metCRC patients. Instead, it suggests that epigenomic profiling and application of FPO could complement the identification of novel combinatorial vulnerabilities to target synchronous ptCRC and mCRC.
© 2024. The Author(s).
Conflict of interest statement
EKHC is a shareholder of Kyan Technologies. The remaining authors declare no competing interests.
Figures






References
LinkOut - more resources
Full Text Sources

