Improved gene therapy for spinal muscular atrophy in mice using codon-optimized hSMN1 transgene and hSMN1 gene-derived promotor
- PMID: 38413838
- PMCID: PMC11018631
- DOI: 10.1038/s44321-024-00037-x
Improved gene therapy for spinal muscular atrophy in mice using codon-optimized hSMN1 transgene and hSMN1 gene-derived promotor
Abstract
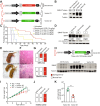
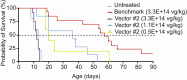
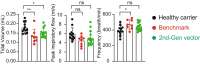
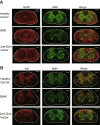
Physiological regulation of transgene expression is a major challenge in gene therapy. Onasemnogene abeparvovec (Zolgensma®) is an approved adeno-associated virus (AAV) vector gene therapy for infants with spinal muscular atrophy (SMA), however, adverse events have been observed in both animals and patients following treatment. The construct contains a native human survival motor neuron 1 (hSMN1) transgene driven by a strong, cytomegalovirus enhancer/chicken β-actin (CMVen/CB) promoter providing high, ubiquitous tissue expression of SMN. We developed a second-generation AAV9 gene therapy expressing a codon-optimized hSMN1 transgene driven by a promoter derived from the native hSMN1 gene. This vector restored SMN expression close to physiological levels in the central nervous system and major systemic organs of a severe SMA mouse model. In a head-to-head comparison between the second-generation vector and a benchmark vector, identical in design to onasemnogene abeparvovec, the 2nd-generation vector showed better safety and improved efficacy in SMA mouse model.
Keywords: AAV; Endogenous Promoter; Gene Therapy; SMA; SMN1.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
QX, HM, GG, and JX are inventors of a patent application filed by the University of Massachusetts Chan Medical School on SMA gene therapy. GG is a scientific co-founder of Voyager Therapeutics, Adrenas Therapeutics, AAVAA Therapeutics, and Aspa Therapeutics and holds equity in these companies. PT, GG, and JX are inventors of patents related to AAV-based gene therapy, some of which were licensed to commercial entities. YM, LJ, FW, JY, and ZK are employees of CANbridge Pharmaceuticals. YM, LJ, GC, JY, and ZK hold equity and/or stock options in CANbridge Pharmaceuticals. The other authors declare no competing interests.
Figures






References
-
- Bernabo P, Tebaldi T, Groen EJN, Lane FM, Perenthaler E, Mattedi F, Newbery HJ, Zhou H, Zuccotti P, Potrich V, et al. In vivo translatome profiling in spinal muscular atrophy reveals a role for SMN protein in ribosome biology. Cell Rep. 2017;21:953–965. doi: 10.1016/j.celrep.2017.10.010. - DOI - PMC - PubMed
-
- Besse A, Astord S, Marais T, Roda M, Giroux B, Lejeune FX, Relaix F, Smeriglio P, Barkats M, Biferi MG. AAV9-mediated expression of SMN restricted to neurons does not rescue the spinal muscular atrophy phenotype in mice. Mol Ther. 2020;28:1887–1901. doi: 10.1016/j.ymthe.2020.05.011. - DOI - PMC - PubMed
-
- Bischof M, Lorenzi M, Lee J, Druyts E, Balijepalli C, Dabbous O. Matching-adjusted indirect treatment comparison of onasemnogene abeparvovec and nusinersen for the treatment of symptomatic patients with spinal muscular atrophy type 1. Curr Med Res Opin. 2021;37:1719–1730. doi: 10.1080/03007995.2021.1947216. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

