Zebrafish tumour xenograft models: a prognostic approach to epithelial ovarian cancer
- PMID: 38413842
- PMCID: PMC10899227
- DOI: 10.1038/s41698-024-00550-9
Zebrafish tumour xenograft models: a prognostic approach to epithelial ovarian cancer
Abstract
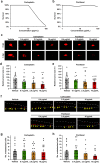
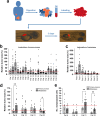
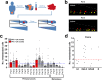
Epithelial ovarian cancer (EOC) is the gynaecological malignancy with highest mortality. Although adjuvant treatment with carboplatin and paclitaxel leads to an objective response in ~80% of these patients, a majority will relapse within two years. Better methods for assessing long-term treatment outcomes are needed. To address this, we established safe and efficacious doses of carboplatin and paclitaxel using IGROV-1 zebrafish-CDX models. Then fluorescently-labelled cell suspensions from 83 tumour biopsies collected at exploratory laparotomy of women with suspected EOC were generated and 37 (45%) were successfully implanted in zebrafish larvae. Among these 19 of 27 pathology-confirmed EOC samples (70%) engrafted. These zebrafish patient-derived tumour xenograft (ZTX) models were treated with carboplatin or paclitaxel and tumour growth/regression and metastatic dissemination were recorded. In a subgroup of nine patients, four ZTX models regressed during carboplatin treatment. All four corresponding patients had >24 months PFS. Furthermore, both ZTX models established from two patients having <24 months PFS failed to regress during carboplatin treatment. Seven of eight models seeding <6 metastatic cells were established from patients having >24 months PFS. In eleven of fourteen patients, FIGO stage I + II or III tumours gave rise to ZTX models seeding <4 or >4 metastatic cells, respectively. In conclusion, ZTX models predicted patients having >24 or <24 months PFS, based on response/no response to carboplatin. Furthermore, high metastatic dissemination in ZTX models correlated to shorter PFS and more advanced disease at diagnosis. These preliminary results suggest that ZTX models could become a useful prognostic tool in EOC treatment planning.
© 2024. The Author(s).
Conflict of interest statement
S.F., M.V., R.A., Z.A., G.V.R., A.E., A.F., and L.J. were or are employees or shareholders in BioReperia AB, a company that is developing ZTX models for diagnosis and prognosis of cancer patients. The other authors declare no conflicts of interest related to this work.
Figures



References
Grants and funding
- Dnr. 2017-01444/VINNOVA (Swedish Governmental Agency for Innovation Systems)
- Dnr. 2017-01444/VINNOVA (Swedish Governmental Agency for Innovation Systems)
- Dnr. 2017-01444/VINNOVA (Swedish Governmental Agency for Innovation Systems)
- MSCA-RISE No. 101007931/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

