Hepatitis B vaccine effectiveness among vaccinated children in Africa: a systematic review and meta-analysis
- PMID: 38413906
- PMCID: PMC10900737
- DOI: 10.1186/s12887-024-04557-w
Hepatitis B vaccine effectiveness among vaccinated children in Africa: a systematic review and meta-analysis
Abstract
Background: Globally, 257 million people have chronic hepatitis. Even though a safe and effective prophylactic vaccine against HBV infection has been available, it causes significant morbidity and mortality. HBV vaccines were designed to improve or modulate the host immune responses. The effectiveness of the vaccine is determined by measuring serum hepatitis B surface antibody (Anti-HBs) level. Therefore, this systematic review aimed to evaluate the effectiveness of hepatitis B vaccine among vaccinated children.
Methods: Preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines was applied for systematically searching of different databases. Only cross-section studies measuring the level of anti-HBs of vaccinated children were included. The seroprotective level with anti-HBs > 10mIU/ml was extracted. The meta-analysis was performed using statistical software for data sciences (STATA) version 14. Effectiveness estimates were reported as a proportion of anti-HBs level. The heterogeneity between studies was evaluated using the I2 test, and I2 > 50% and/or P < 0.10 was considered significant heterogeneity. Significant publication bias was considered when Egger's test P-value < 0.10. The new castle Ottawa scale was used to assess the quality of the studies.
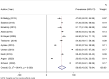
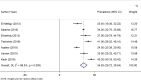
Results: A pooled sample size of the included papers for meta-analysis was 7430. The pooled prevalence of seroprotected children was 56.95%, with a heterogeneity index (I2) of 99.4% (P < 0.001). 35% of the participants were hypo-responders (10-99mIU/ml) and 21.46% were good responders (> 100mIU/ml). Based on subgroup analysis using country of studies conducted, the highest prevalence of anti-HBs was 87.00% (95% CI: 84.56, 89.44), in South Africa, and the lowest was 51.99% (95% CI: 20.41-83.58), with a heterogeneity index I2 = 70.7% (p = 0.009) in Ethiopia.
Conclusion and recommendations: Hepatitis B vaccine seroprotective level in the current pooled analysis have suboptimal, which failed to demonstrate consistent effectiveness for global hepatitis B virus elimination plan in 2030. Using consistent age group may have a significant value for the decision of the HB vaccine effectiveness. A significant heterogeneity was observed both in studies conducted in Ethiopia and Egypt. Therefore, the impact of HB vaccination on the prevention of hepatitis B virus infection should be assessed regularly in those countries. Future meta-analysis is needed to investigate all possible vaccines in a separate way of reviewing, which will lead to a strong conclusion and recommendations.
Keywords: Africa; Anti-hepatitis B surface; Hepatitis B vaccine; Hepatitis B virus; Meta-analysis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Glucocorticosteroids for people with alcoholic hepatitis.Cochrane Database Syst Rev. 2017 Nov 2;11(11):CD001511. doi: 10.1002/14651858.CD001511.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Apr 09;4:CD001511. doi: 10.1002/14651858.CD001511.pub4. PMID: 29096421 Free PMC article. Updated.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
Cited by
-
Advancing Human Vaccine Development Using Humanized Mouse Models.Vaccines (Basel). 2024 Sep 4;12(9):1012. doi: 10.3390/vaccines12091012. Vaccines (Basel). 2024. PMID: 39340042 Free PMC article. Review.
-
Hepatitis B virus infection and its treatment in Eastern Ethiopia.World J Hepatol. 2025 Jan 27;17(1):99209. doi: 10.4254/wjh.v17.i1.99209. World J Hepatol. 2025. PMID: 39871910 Free PMC article.
-
Cost-effectiveness of tenofovir disoproxil fumarate prophylaxis for perinatal hepatitis B virus prevention in Ethiopia: a decision analytical modeling.BMC Public Health. 2025 Apr 7;25(1):1300. doi: 10.1186/s12889-025-22498-6. BMC Public Health. 2025. PMID: 40197306 Free PMC article.
-
Prospects for Controlling Hepatitis B Globally.Pathogens. 2024 Mar 29;13(4):291. doi: 10.3390/pathogens13040291. Pathogens. 2024. PMID: 38668246 Free PMC article. Review.
-
Hepatitis B Elimination Globally: The Answer May Not Be the Same for Everyone.J Pediatric Infect Dis Soc. 2024 Nov 21;13(Supplement_5):S139-S141. doi: 10.1093/jpids/piae067. J Pediatric Infect Dis Soc. 2024. PMID: 38943670 Free PMC article.
References
-
- World Health Organization (WHO). Hepatitis B. Fact sheet. Accessed in July 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

