GOIZ ZAINDU study: a FINGER-like multidomain lifestyle intervention feasibility randomized trial to prevent dementia in Southern Europe
- PMID: 38413990
- PMCID: PMC10898038
- DOI: 10.1186/s13195-024-01393-z
GOIZ ZAINDU study: a FINGER-like multidomain lifestyle intervention feasibility randomized trial to prevent dementia in Southern Europe
Abstract
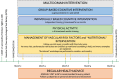
Background: GOIZ ZAINDU ("caring early" in Basque) is a pilot study to adapt the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) methodology to the Basque population and evaluate the feasibility and adherence to a FINGER-like multidomain intervention program. Additional aims included the assessment of efficacy on cognition and data collection to design a large efficacy trial.
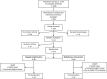
Method: GOIZ ZAINDU is a 1-year, randomized, controlled trial of a multidomain intervention in persons aged 60+ years, with Cardiovascular Risk Factors, Aging and Dementia (CAIDE) risk score ≥ 6, no diagnosis of dementia, and below-than-expected performance in at least one of three cognitive screening tests. Randomization to a multidomain intervention (MD-Int) or regular health advice (RHA) was stratified by sex, age (>/≤ 75), and cognitive status (mild cognitive impairment (MCI)/normal cognition). MD-Int included cardiovascular risk factor control, nutritional counseling, physical activity, and cognitive training. The primary outcomes were retention rate and adherence to the intervention program. Exploratory cognitive outcomes included changes in the Neuropsychological Test Battery z-scores. Analyses were performed according to the intention to treat.
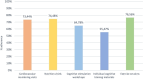
Results: One hundred twenty-five participants were recruited (mean age: 75.64 (± 6.46); 58% women). The MD-Int (n = 61) and RHA (n = 64) groups were balanced in terms of their demographics and cognition. Fifty-two (85%) participants from the RHA group and 56 (88%) from the MD-Int group completed the study. More than 70% of the participants had high overall adherence to the intervention activities. The risk of cognitive decline was higher in the RHA group than in the MD-Int group in terms of executive function (p =.019) and processing speed scores (p =.026).
Conclusions: The GOIZ-ZAINDU study proved that the FINGER methodology is adaptable and feasible in a different socio-cultural environment. The exploratory efficacy results showed a lower risk of decline in executive function and processing speed in the intervention group. These results support the design of a large-scale efficacy trial.
Trial registration: GOIZ ZAINDU feasibility trial was approved and registered by the Euskadi Drug Research Ethics Committee (ID: PI2017134) on 23 January 2018. Retrospectively registered in ClinicalTrials.gov (NCT06163716) on 8 December 2023.
Keywords: Cognitive impairment; Dementia prevention; Lifestyle; Multidomain intervention; Randomized trial.
© 2024. The Author(s).
Conflict of interest statement
MK is Editor-in-Chief of the journal “Alzheimer’s Research and Therapy”.
Figures
References
-
- Collaborators G 2016 D. Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. - DOI - PMC - PubMed
-
- Solomon A, Kivipelto M, Soininen H. Prevention of Alzheimer’s disease: moving backward through the lifespan. J Alzheimer’s Dis. 2013;33(Suppl 1):S465–9. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical