Protocol for a single-arm, pilot trial of creatine monohydrate supplementation in patients with Alzheimer's disease
- PMID: 38414003
- PMCID: PMC10898014
- DOI: 10.1186/s40814-024-01469-5
Protocol for a single-arm, pilot trial of creatine monohydrate supplementation in patients with Alzheimer's disease
Abstract
Background: Impaired brain bioenergetics is a pathological hallmark of Alzheimer's disease (AD) and is a compelling target for AD treatment. Patients with AD exhibit dysfunction in the brain creatine (Cr) system, which is integral in maintaining bioenergetic flux. Recent studies in AD mouse models suggest Cr supplementation improves brain mitochondrial function and may be protective of AD peptide pathology and cognition.
Aims: The Creatine to Augment Bioenergetics in Alzheimer's disease (CABA) study is designed to primarily assess the feasibility of supplementation with 20 g/day of creatine monohydrate (CrM) in patients with cognitive impairment due to AD. Secondary aims are designed to generate preliminary data investigating changes in brain Cr levels, cognition, peripheral and brain mitochondrial function, and muscle strength and size.
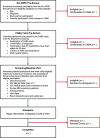
Methods: CABA is an 8-week, single-arm pilot study that will recruit 20 patients with cognitive impairment due to AD. Participants attend five in-person study visits: two visits at baseline to conduct screening and baseline assessments, a 4-week visit, and two 8-week visits. Outcomes assessment includes recruitment, retention, and compliance, cognitive testing, magnetic resonance spectroscopy of brain metabolites, platelet and lymphocyte mitochondrial function, and muscle strength and morphology at baseline and 8 weeks.
Discussion: CABA is the first study to investigate CrM as a potential treatment in patients with AD. The pilot data generated by this study are pertinent to inform the design of future large-scale efficacy trials.
Trial registration: ClinicalTrials.gov, NCT05383833 , registered on 20 May 2022.
Keywords: Alzheimer’s disease; Bioenergetics; Brain; Creatine; Pilot trial.
© 2024. The Author(s).
Conflict of interest statement
M. K. T. receives research support from the National Institutes of Health (NIH) and the Alzheimer’s Association. In the past 2 years, J. M. B. has received research support from the NIH; research support to conduct clinical trials (paid to institution) from Eli Lilly, Amylyx Pharmaceuticals, Biogen, AbbVie, Astra-Zeneca, and Roche; and has served as a consultant for Renew Research, Amylyx Pharmaceuticals, Eisai, and Eli Lilly. I. Y. C. receives research support from the NIH and the US Highbush Blueberry Council. T. J. H. receives support from the National Strength and Conditioning Association. P. L. receives research support from the NIH. A. N. S. declares no competing interests. D. K. S. receives research support from the NIH, the Egg Nutrition Center, and the US Highbush Blueberry Council. R. H. S. receives research support from the NIH. H. M. W. receives research support from the NIH and the Alzheimer’s Association.
Similar articles
-
Creatine monohydrate pilot in Alzheimer's: Feasibility, brain creatine, and cognition.Alzheimers Dement (N Y). 2025 May 19;11(2):e70101. doi: 10.1002/trc2.70101. eCollection 2025 Apr-Jun. Alzheimers Dement (N Y). 2025. PMID: 40395689 Free PMC article.
-
Creatine as a Therapeutic Target in Alzheimer's Disease.Curr Dev Nutr. 2023 Sep 29;7(11):102011. doi: 10.1016/j.cdnut.2023.102011. eCollection 2023 Nov. Curr Dev Nutr. 2023. PMID: 37881206 Free PMC article. Review.
-
A pilot protocol to assess the feasibility of a virtual multiple crossover, randomized controlled trial design using methylphenidate in mild cognitive impairment.Trials. 2020 Dec 11;21(1):1016. doi: 10.1186/s13063-020-04752-x. Trials. 2020. PMID: 33308285 Free PMC article.
-
A 12-month randomised pilot trial of the Alzheimer's and music therapy study: a feasibility assessment of music therapy and physical activity in patients with mild-to-moderate Alzheimer's disease.Pilot Feasibility Stud. 2023 Apr 19;9(1):61. doi: 10.1186/s40814-023-01287-1. Pilot Feasibility Stud. 2023. PMID: 37076884 Free PMC article.
-
Souvenaid for Alzheimer's disease.Cochrane Database Syst Rev. 2020 Dec 15;12(12):CD011679. doi: 10.1002/14651858.CD011679.pub2. Cochrane Database Syst Rev. 2020. PMID: 33320335 Free PMC article.
Cited by
-
Creatine monohydrate pilot in Alzheimer's: Feasibility, brain creatine, and cognition.Alzheimers Dement (N Y). 2025 May 19;11(2):e70101. doi: 10.1002/trc2.70101. eCollection 2025 Apr-Jun. Alzheimers Dement (N Y). 2025. PMID: 40395689 Free PMC article.
References
-
- Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RYK, Lannfelt L, Bradley H, Rabe M, Koyama A, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Abeta protofibril antibody. Alzheimers Res Ther. 2021;13(1):80. doi: 10.1186/s13195-021-00813-8. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical