Protocol for a single-arm, pilot trial of creatine monohydrate supplementation in patients with Alzheimer's disease
- PMID: 38414003
- PMCID: PMC10898014
- DOI: 10.1186/s40814-024-01469-5
Protocol for a single-arm, pilot trial of creatine monohydrate supplementation in patients with Alzheimer's disease
Abstract
Background: Impaired brain bioenergetics is a pathological hallmark of Alzheimer's disease (AD) and is a compelling target for AD treatment. Patients with AD exhibit dysfunction in the brain creatine (Cr) system, which is integral in maintaining bioenergetic flux. Recent studies in AD mouse models suggest Cr supplementation improves brain mitochondrial function and may be protective of AD peptide pathology and cognition.
Aims: The Creatine to Augment Bioenergetics in Alzheimer's disease (CABA) study is designed to primarily assess the feasibility of supplementation with 20 g/day of creatine monohydrate (CrM) in patients with cognitive impairment due to AD. Secondary aims are designed to generate preliminary data investigating changes in brain Cr levels, cognition, peripheral and brain mitochondrial function, and muscle strength and size.
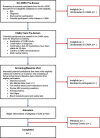
Methods: CABA is an 8-week, single-arm pilot study that will recruit 20 patients with cognitive impairment due to AD. Participants attend five in-person study visits: two visits at baseline to conduct screening and baseline assessments, a 4-week visit, and two 8-week visits. Outcomes assessment includes recruitment, retention, and compliance, cognitive testing, magnetic resonance spectroscopy of brain metabolites, platelet and lymphocyte mitochondrial function, and muscle strength and morphology at baseline and 8 weeks.
Discussion: CABA is the first study to investigate CrM as a potential treatment in patients with AD. The pilot data generated by this study are pertinent to inform the design of future large-scale efficacy trials.
Trial registration: ClinicalTrials.gov, NCT05383833 , registered on 20 May 2022.
Keywords: Alzheimer’s disease; Bioenergetics; Brain; Creatine; Pilot trial.
© 2024. The Author(s).
Conflict of interest statement
M. K. T. receives research support from the National Institutes of Health (NIH) and the Alzheimer’s Association. In the past 2 years, J. M. B. has received research support from the NIH; research support to conduct clinical trials (paid to institution) from Eli Lilly, Amylyx Pharmaceuticals, Biogen, AbbVie, Astra-Zeneca, and Roche; and has served as a consultant for Renew Research, Amylyx Pharmaceuticals, Eisai, and Eli Lilly. I. Y. C. receives research support from the NIH and the US Highbush Blueberry Council. T. J. H. receives support from the National Strength and Conditioning Association. P. L. receives research support from the NIH. A. N. S. declares no competing interests. D. K. S. receives research support from the NIH, the Egg Nutrition Center, and the US Highbush Blueberry Council. R. H. S. receives research support from the NIH. H. M. W. receives research support from the NIH and the Alzheimer’s Association.
References
-
- Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RYK, Lannfelt L, Bradley H, Rabe M, Koyama A, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Abeta protofibril antibody. Alzheimers Res Ther. 2021;13(1):80. doi: 10.1186/s13195-021-00813-8. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical