Targeting HDAC6 improves anti-CD47 immunotherapy
- PMID: 38414061
- PMCID: PMC10898070
- DOI: 10.1186/s13046-024-02982-4
Targeting HDAC6 improves anti-CD47 immunotherapy
Abstract
Background: Cancer cells can overexpress CD47, an innate immune checkpoint that prevents phagocytosis upon interaction with signal regulatory protein alpha (SIRPα) expressed in macrophages and other myeloid cells. Several clinical trials have reported that CD47 blockade reduces tumor growth in hematological malignancies. However, CD47 blockade has shown modest results in solid tumors, including melanoma. Our group has demonstrated that histone deacetylase 6 inhibitors (HDAC6is) have immunomodulatory properties, such as controlling macrophage phenotype and inflammatory properties. However, the molecular and cellular mechanisms controlling these processes are not fully understood. In this study, we evaluated the role of HDAC6 in regulating the CD47/SIRPα axis and phagocytosis in macrophages.
Methods: We tested the role of HDAC6is, especially Nexturastat A, in regulating macrophage phenotype and phagocytic function using bone marrow-derived macrophages and macrophage cell lines. The modulation of the CD47/SIRPα axis and phagocytosis by HDAC6is was investigated using murine and human melanoma cell lines and macrophages. Phagocytosis was evaluated via coculture assays of macrophages and melanoma cells by flow cytometry and immunofluorescence. Lastly, to evaluate the antitumor activity of Nexturastat A in combination with anti-CD47 or anti-SIRPα antibodies, we performed in vivo studies using the SM1 and/or B16F10 melanoma mouse models.
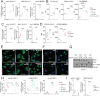
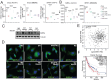
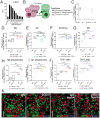
Results: We observed that HDAC6is enhanced the phenotype of antitumoral M1 macrophages while decreasing the protumoral M2 phenotype. In addition, HDAC6 inhibition diminished the expression of SIRPα, increased the expression of other pro-phagocytic signals in macrophages, and downregulated CD47 expression in mouse and human melanoma cells. This regulatory role on the CD47/SIRPα axis translated into enhanced antitumoral phagocytic capacity of macrophages treated with Nexturastat A and anti-CD47. We also observed that the systemic administration of HDAC6i enhanced the in vivo antitumor activity of anti-CD47 blockade in melanoma by modulating macrophage and natural killer cells in the tumor microenvironment. However, Nexturastat A did not enhance the antitumor activity of anti-SIRPα despite its modulation of macrophage populations in the SM1 tumor microenvironment.
Conclusions: Our results demonstrate the critical regulatory role of HDAC6 in phagocytosis and innate immunity for the first time, further underscoring the use of these inhibitors to potentiate CD47 immune checkpoint blockade therapeutic strategies.
Keywords: CD47; Histone deacetylases; Immunotherapy; Macrophages; Melanoma; Nexturastat A; Phagocytosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Strome SE, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

