Organoids as a biomarker for personalized treatment in metastatic colorectal cancer: drug screen optimization and correlation with patient response
- PMID: 38414064
- PMCID: PMC10898042
- DOI: 10.1186/s13046-024-02980-6
Organoids as a biomarker for personalized treatment in metastatic colorectal cancer: drug screen optimization and correlation with patient response
Abstract
Background: The inability to predict treatment response of colorectal cancer patients results in unnecessary toxicity, decreased efficacy and survival. Response testing on patient-derived organoids (PDOs) is a promising biomarker for treatment efficacy. The aim of this study is to optimize PDO drug screening methods for correlation with patient response and explore the potential to predict responses to standard chemotherapies.
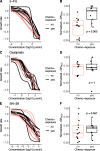
Methods: We optimized drug screen methods on 5-11 PDOs per condition of the complete set of 23 PDOs from patients treated for metastatic colorectal cancer (mCRC). PDOs were exposed to 5-fluorouracil (5-FU), irinotecan- and oxaliplatin-based chemotherapy. We compared medium with and without N-acetylcysteine (NAC), different readouts and different combination treatment set-ups to capture the strongest association with patient response. We expanded the screens using the optimized methods for all PDOs. Organoid sensitivity was correlated to the patient's response, determined by % change in the size of target lesions. We assessed organoid sensitivity in relation to prior exposure to chemotherapy, mutational status and sidedness.
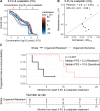
Results: Drug screen optimization involved excluding N-acetylcysteine from the medium and biphasic curve fitting for 5-FU & oxaliplatin combination screens. CellTiter-Glo measurements were comparable with CyQUANT and did not affect the correlation with patient response. Furthermore, the correlation improved with application of growth rate metrics, when 5-FU & oxaliplatin was screened in a ratio, and 5-FU & SN-38 using a fixed dose of SN-38. Area under the curve was the most robust drug response curve metric. After optimization, organoid and patient response showed a correlation coefficient of 0.58 for 5-FU (n = 6, 95% CI -0.44,0.95), 0.61 for irinotecan- (n = 10, 95% CI -0.03,0.90) and 0.60 for oxaliplatin-based chemotherapy (n = 11, 95% CI -0.01,0.88). Median progression-free survival of patients with resistant PDOs to oxaliplatin-based chemotherapy was significantly shorter than sensitive PDOs (3.3 vs 10.9 months, p = 0.007). Increased resistance to 5-FU in patients with prior exposure to 5-FU/capecitabine was adequately reflected in PDOs (p = 0.003).
Conclusions: Our study emphasizes the critical impact of the screening methods for determining correlation between PDO drug screens and mCRC patient outcomes. Our 5-step optimization strategy provides a basis for future research on the clinical utility of PDO screens.
Keywords: Cancer; Chemotherapy; Colorectal cancer; Drug screening; Oncology; Organoids; Precision medicine.
© 2024. The Author(s).
Conflict of interest statement
Miriam Koopman reports institutional financial interests with Amgen, Bayer, Bristol-Myers Squibb, Merck-Serono, Nordic Pharma, Pierre Fabre, Servier, Sirtex, Roche, Sanofi, and Personal Genome Diagnostics. Miriam Koopman reports the following non-financial interests: an advisory role for ZON-MW, membership of the scientific board of the Dutch Cancer Society (KWF), chairmanship of the Dutch Colorectal Cancer Group (DCCG), and principal investigator (PI) of the Prospective Dutch Colorectal Cancer (PLCRC) cohort. Jeanine Roodhart reports institutional financial interests with BMS, Pierre Fabre, Servier, Cleara, Xilis, DoMore diagnostics and HUB organoids B.V. Jeanine Roodhart reports an advisory role for Bayer, BMS, Merck-Serono, Pierre Fabre, Servier, AMGEN and board member of Foundation Hubrecht Organoid Biobank. Liselot Valkenburg-van Iersel reports financial interests with and an advisory role for Servier and Pierre Fabre. All other authors declare that they have no competing interests.
Figures



References
-
- Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G) Ann Oncol. 2016;27(8):1539–1546. - PubMed
-
- Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with Cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal Cancer: a randomized clinical trial. JAMA. 2017;317(23):2392–2401. - PMC - PubMed
-
- Kwakman JJM, Simkens LHJ, van Rooijen JM, van de Wouw AJ, Ten Tije AJ, Creemers GJM, et al. Randomized phase III trial of S-1 versus capecitabine in the first-line treatment of metastatic colorectal cancer: SALTO study by the Dutch colorectal Cancer group. Ann Oncol. 2017;28(6):1288–1293. - PubMed
-
- Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with Nivolumab plus Ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal Cancer. J Clin Oncol. 2018;36(8):773–779. - PubMed
-
- Sawyers CL. The cancer biomarker problem. Nature. 2008;452(7187):548–552. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

