Impact of tissue factor expression and administration routes on thrombosis development induced by mesenchymal stem/stromal cell infusions: re-evaluating the dogma
- PMID: 38414067
- PMCID: PMC10900728
- DOI: 10.1186/s13287-023-03582-3
Impact of tissue factor expression and administration routes on thrombosis development induced by mesenchymal stem/stromal cell infusions: re-evaluating the dogma
Abstract
Background: Hyperactive coagulation might cause dangerous complications such as portal vein thrombosis and pulmonary embolism after mesenchymal stem/stromal cell (MSC) therapy. Tissue factor (TF), an initiator of the extrinsic coagulation pathway, has been suggested as a predictor of this process.
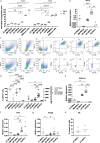
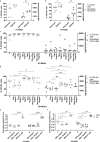
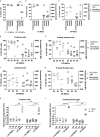
Methods: The expression of TF and other pro- and anticoagulant genes was analyzed in xeno- and serum-free manufactured MSCs. Furthermore, culture factors affecting its expression in MSCs were investigated. Finally, coagulation tests of fibrinogen, D-dimer, aPPTs, PTs, and TTs were measured in patient serum after umbilical cord (UC)-MSC infusions to challenge a potential connection between TF expression and MSC-induced coagulant activity. RESULTS: Xeno- and serum-free cultured adipose tissue and UC-derived MSCs expressed the highest level of TF, followed by those from dental pulp, and the lowest expression was observed in MSCs of bone marrow origin. Environmental factors such as cell density, hypoxia, and inflammation impact TF expression, so in vitro analysis might fail to reflect their in vivo behaviors. MSCs also expressed heterogeneous levels of the coagulant factor COL1A1 and surface phosphatidylserine and anticoagulant factors TFPI and PTGIR. MSCs of diverse origins induced fibrin clots in healthy plasma that were partially suppressed by an anti-TF inhibitory monoclonal antibody. Furthermore, human umbilical vein endothelial cells exhibited coagulant activity in vitro despite their negative expression of TF and COL1A1. Patients receiving intravenous UC-MSC infusion exhibited a transient increase in D-dimer serum concentration, while this remained stable in the group with intrathecal infusion. There was no correlation between TF expression and D-dimer or other coagulation indicators.
Conclusions: The study suggests that TF cannot be used as a solid biomarker to predict MSC-induced hypercoagulation. Local administration, prophylactic intervention with anticoagulation drugs, and monitoring of coagulation indicators are useful to prevent thrombogenic events in patients receiving MSCs. Trial registration NCT05292625. Registered March 23, 2022, retrospectively registered, https://www.
Clinicaltrials: gov/ct2/show/NCT05292625?term=NCT05292625&draw=2&rank=1 . NCT04919135. Registered June 9, 2021, https://www.
Clinicaltrials: gov/ct2/show/NCT04919135?term=NCT04919135&draw=2&rank=1 .
Keywords: Cell therapy; Coagulation; Mesenchymal stem cell; Mesenchymal stromal cell; Thrombosis; Tissue factor; Umbilical cord.
© 2024. The Author(s).
Conflict of interest statement
V.T.H., D.M.H., T.K.N., P.T.K. T, L.A.T.N., T.L.N., B.V.A., and L.N.T. are employed by the not-for-profit Vinmec Health Care System.
Figures





Similar articles
-
Lethal pulmonary thromboembolism in mice induced by intravenous human umbilical cord mesenchymal stem cell-derived large extracellular vesicles in a dose- and tissue factor-dependent manner.Acta Pharmacol Sin. 2024 Nov;45(11):2300-2312. doi: 10.1038/s41401-024-01327-3. Epub 2024 Jun 24. Acta Pharmacol Sin. 2024. PMID: 38914677
-
Cell Sheets Formation Enhances Therapeutic Effects of Human Umbilical Cord Mesenchymal Stem Cells on Spinal Cord Injury.CNS Neurosci Ther. 2024 Dec;30(12):e70163. doi: 10.1111/cns.70163. CNS Neurosci Ther. 2024. PMID: 39670537 Free PMC article.
-
Human Infrapatellar Fat Pad Mesenchymal Stem Cell-derived Extracellular Vesicles Purified by Anion Exchange Chromatography Suppress Osteoarthritis Progression in a Mouse Model.Clin Orthop Relat Res. 2024 Jul 1;482(7):1246-1262. doi: 10.1097/CORR.0000000000003067. Epub 2024 Apr 19. Clin Orthop Relat Res. 2024. PMID: 38662932 Free PMC article.
-
Systematic review and meta-analysis of mesenchymal stromal/stem cells as strategical means for the treatment of COVID-19.Ther Adv Respir Dis. 2023 Jan-Dec;17:17534666231158276. doi: 10.1177/17534666231158276. Ther Adv Respir Dis. 2023. PMID: 37128999 Free PMC article.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
Cited by
-
Tissue Engineering and Regenerative Medicine: Perspectives and Challenges.MedComm (2020). 2025 Apr 24;6(5):e70192. doi: 10.1002/mco2.70192. eCollection 2025 May. MedComm (2020). 2025. PMID: 40290901 Free PMC article. Review.
-
Calcium Chloride vs. Mechanical Preparation of Fibrinogen-Depleted Human Platelet Lysate: Implications for Umbilical Cord Mesenchymal Stem Cell Culture.Life (Basel). 2024 Dec 27;15(1):12. doi: 10.3390/life15010012. Life (Basel). 2024. PMID: 39859952 Free PMC article.
-
Recent Stem-Cell-Based and Stem-Cell-Free Possibilities for the Therapeutic Management of the Osteonecrosis of the Jaw.Biomolecules. 2025 Apr 16;15(4):595. doi: 10.3390/biom15040595. Biomolecules. 2025. PMID: 40305370 Free PMC article. Review.
-
Microglia Morphological Response to Mesenchymal Stromal Cell Extracellular Vesicles Demonstrates EV Therapeutic Potential for Modulating Neuroinflammation.bioRxiv [Preprint]. 2024 Jul 3:2024.07.01.601612. doi: 10.1101/2024.07.01.601612. bioRxiv. 2024. Update in: J Biol Eng. 2024 Oct 17;18(1):58. doi: 10.1186/s13036-024-00449-w. PMID: 39005342 Free PMC article. Updated. Preprint.
-
Microglia morphological response to mesenchymal stromal cell extracellular vesicles demonstrates EV therapeutic potential for modulating neuroinflammation.J Biol Eng. 2024 Oct 17;18(1):58. doi: 10.1186/s13036-024-00449-w. J Biol Eng. 2024. PMID: 39420399 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

