Real-time single-molecule imaging of CaMKII-calmodulin interactions
- PMID: 38414237
- PMCID: PMC11630639
- DOI: 10.1016/j.bpj.2024.02.021
Real-time single-molecule imaging of CaMKII-calmodulin interactions
Abstract
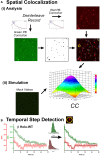
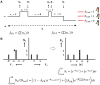
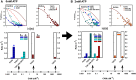
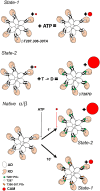
The binding of calcium/calmodulin (CAM) to calcium/calmodulin-dependent protein kinase II (CaMKII) initiates an ATP-driven cascade that triggers CaMKII autophosphorylation. The autophosphorylation in turn increases the CaMKII affinity for CAM. Here, we studied the ATP dependence of CAM association with the actin-binding CaMKIIβ isoform using single-molecule total internal reflection fluorescence microscopy. Rhodamine-CAM associations/dissociations to surface-immobilized Venus-CaMKIIβ were resolved with 0.5 s resolution from video records, batch-processed with a custom algorithm. CAM occupancy was determined simultaneously with spot-photobleaching measurement of CaMKII holoenzyme stoichiometry. We show the ATP-dependent increase of the CAM association requires dimer formation for both the α and β isoforms. The study of mutant β holoenzymes revealed that the ATP-dependent increase in CAM affinity results in two distinct states. The phosphorylation-defective (T287.306-307A) holoenzyme resides only in the low-affinity state. CAM association is further reduced in the T287A holoenzyme relative to T287.306-307A. In the absence of ATP, the affinity of CAM for the T287.306-307A mutant and the wild-type monomer are comparable. The affinity of the ATP-binding impaired (K43R) mutant is even weaker. In ATP, the K43R holoenzyme resides in the low-affinity state. The phosphomimetic mutant (T287D) resides only in a 1000-fold higher-affinity state, with mean CAM occupancy of more than half of the 14-mer holoenzyme stoichiometry in picomolar CAM. ATP promotes T287D holoenzyme disassembly but does not elevate CAM occupancy. Single Poisson distributions characterized the ATP-dependent CAM occupancy of mutant holoenzymes. In contrast, the CAM occupancy of the wild-type population had a two-state distribution with both low- and high-affinity states represented. The low-affinity state was the dominant state, a result different from published in vitro assays. Differences in assay conditions can alter the balance between activating and inhibitory autophosphorylation. Bound ATP could be sufficient for CaMKII structural function, while antagonistic autophosphorylations may tune CaMKII kinase-regulated action-potential frequency decoding in vivo.
Copyright © 2024 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Similar articles
-
Flexible linkers in CaMKII control the balance between activating and inhibitory autophosphorylation.Elife. 2020 Mar 9;9:e53670. doi: 10.7554/eLife.53670. Elife. 2020. PMID: 32149607 Free PMC article.
-
Architectural Dynamics of CaMKII-Actin Networks.Biophys J. 2019 Jan 8;116(1):104-119. doi: 10.1016/j.bpj.2018.11.006. Epub 2018 Nov 10. Biophys J. 2019. PMID: 30527447 Free PMC article.
-
Deciphering CaMKII Multimerization Using Fluorescence Correlation Spectroscopy and Homo-FRET Analysis.Biophys J. 2017 Mar 28;112(6):1270-1281. doi: 10.1016/j.bpj.2017.02.005. Biophys J. 2017. PMID: 28355553 Free PMC article.
-
CASK and CaMKII function in Drosophila memory.Front Neurosci. 2014 Jun 25;8:178. doi: 10.3389/fnins.2014.00178. eCollection 2014. Front Neurosci. 2014. PMID: 25009461 Free PMC article. Review.
-
Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function.Annu Rev Biochem. 2002;71:473-510. doi: 10.1146/annurev.biochem.71.110601.135410. Epub 2001 Nov 9. Annu Rev Biochem. 2002. PMID: 12045104 Review.
Cited by
-
Hub stability in the calcium calmodulin-dependent protein kinase II.Commun Biol. 2024 Jun 25;7(1):766. doi: 10.1038/s42003-024-06423-y. Commun Biol. 2024. PMID: 38918547 Free PMC article.
References
-
- Zhang M., Tanaka T., Ikura M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 1995;2:758–767. - PubMed
-
- De Koninck P., Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science (New York, N.Y. 1998;279:227–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

